Products may be shipped at a different temperature than the recommended long-term storage temperature. If the product quality is sensitive to short-term exposure to conditions other than the recommended long-term storage, it will be shipped on wet or dry-ice. If the product quality is NOT affected by short-term exposure to conditions other than the recommended long-term storage, it will be shipped at ambient temperature. As shipping routes are configured for minimum transit times, shipping at ambient temperature helps control shipping costs for our customers. For more information, please refer to the Storage and Transport Conditions document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/316/622/storage-transport-conditions-mk.pdf
417556
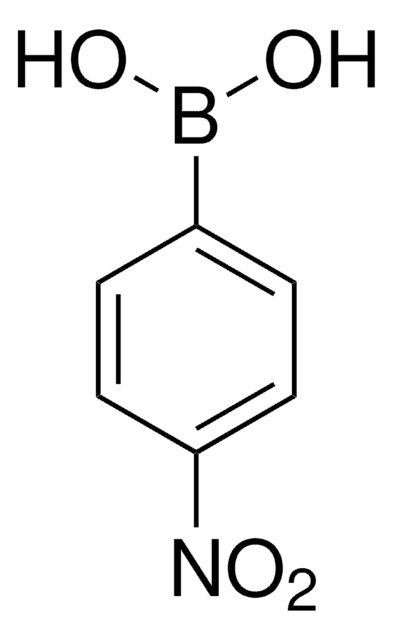
4-Fluorophenylboronic acid
≥95%
Sinonimo/i:
(4-Fluorophenyl)boric acid, (4-Fluorophenyl)dihydroxyborane, (4-Fluorophenyl)dihydroxyboron, (p-Fluorophenyl)boric acid, 4-Fluorobenzeneboronic acid, p-Fluorobenzylboronic acid, p-Fluorophenylboronic acid, NSC 142683
Scegli un formato
23,38 €
Prezzo di listino33,40 €Risparmia il 30%Scegli un formato
About This Item
23,38 €
Prezzo di listino33,40 €Risparmia il 30%Prodotti consigliati
Livello qualitativo
Saggio
≥95%
Stato
powder
Punto di fusione
262-265 °C (lit.)
Gruppo funzionale
fluoro
Stringa SMILE
OB(O)c1ccc(F)cc1
InChI
1S/C6H6BFO2/c8-6-3-1-5(2-4-6)7(9)10/h1-4,9-10H
LBUNNMJLXWQQBY-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Applicazioni
It can also be used as a reactant in:
- Suzuki coupling using microwave and triton B catalyst.[4]
- Pd-catalyzed direct arylation of pyrazoles with phenylboronic acids.[5]
- Mizoroki-Heck and Suzuki-Miyaura coupling reactions catalyzed by palladium nanoparticles.[6]
- Cu-catalyzed Petasis reactions.[7]
- Tandem-type Pd(II)-catalyzed oxidative Heck reaction and intramolecular C-H amidation sequence.[8]
- Ruthenium catalyzed direct arylation.[9]
- Rh-catalyzed asymmetric conjugate additions.[10]
- Ligand-free copper-catalyzed coupling of nitro arenes with arylboronic acids.[11]
- Regioselective arylation and alkynylation by Suzuki-Miyaura and Sonogashira cross-coupling reactions.[12]
- Suzuki cross-coupling of tetrabromothiophene.[13]
- Palladium-catalyzed addition to nitriles.[14]
Altre note
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
-
How is shipping temperature determined? And how is it related to the product storage temperature?
1 risposta-
Utile?
-
-
How can I determine the shelf life / expiration / retest date of this product?
1 risposta-
If this product has an expiration or retest date, it will be shown on the Certificate of Analysis (COA, CofA). If there is no retest or expiration date listed on the product's COA, we do not have suitable stability data to determine a shelf life. For these products, the only date on the COA will be the release date; a retest, expiration, or use-by-date will not be displayed.
For all products, we recommend handling per defined conditions as printed in our product literature and website product descriptions. We recommend that products should be routinely inspected by customers to ensure they perform as expected.
For products without retest or expiration dates, our standard warranty of 1 year from the date of shipment is applicable.
For more information, please refer to the Product Dating Information document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/449/386/product-dating-information-mk.pdfUtile?
-
Filtri attivi
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.







![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)