192694
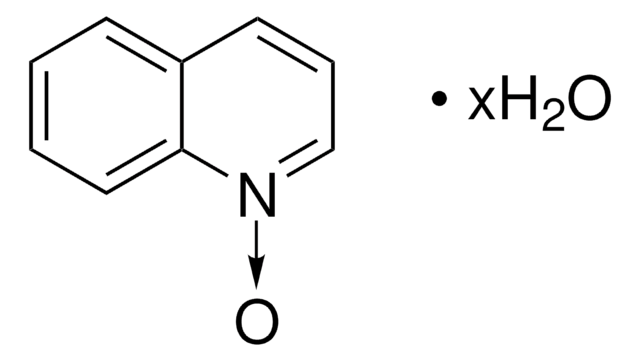
Isoquinoline N-oxide
98%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C9H7NO
Numero CAS:
Peso molecolare:
145.16
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
98%
Forma fisica
solid
Punto di fusione
103-105 °C (lit.)
Stringa SMILE
[O-][n+]1ccc2ccccc2c1
InChI
1S/C9H7NO/c11-10-6-5-8-3-1-2-4-9(8)7-10/h1-7H
RZIAABRFQASVSW-UHFFFAOYSA-N
Descrizione generale
Photochemical isomerization of isoquinoline N-oxide in methanol or water has been investigated by steady-light irradiation and flash spectroscopy. It is a useful intermediate for isoquinoline derivatives. It causes the oxidation of fullerene C60 under ultrasonic irradiation in air.
Applicazioni
Isoquinoline N-oxide was used in the synthesis of N-alkoxy isoquinolinium and N-alkoxy 4-phenylpyridinium ion terminated polystyrenes.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Iodine-mediated electrophilic cyclization of 2-alkynylbenzaldoximes leading to the formation of iodoisoquinoline N-oxides.
Huo Z, et al.
Tetrahedron Letters, 49(38), 5531-5533 (2008)
Weon-Bae Ko et al.
Ultrasonics, 42(1-9), 611-615 (2004-03-30)
The reaction of C60 with various amine N-oxides such as 3-picoline N-oxide (Aldrich 98.0%), pyridine N-oxide hydrate (Aldrich 95.0%), quinoline N-oxide (Aldrich 97.0%), isoquinoline N-oxide (Aldrich 98.0%) under ultrasonic irradiation in air at 25-43 degrees C causes the oxidation of
N-alkoxy pyridinium ion terminated polystyrenes: A facile route to photoinduced block copolymerization.
Durmaz YY, et al.
Journal of Polymer Science Part A: Polymer Chemistry, 45(3), 423-428 (2007)
The primary photochemical process of isoquinoline N-oxide in hydroxylic solvents.
Ono I and Hata N.
Bulletin of the Chemical Society of Japan, 46, 3658-3662 (1973)
Oleg V Larionov et al.
Organic letters, 16(3), 864-867 (2014-01-15)
A one-step transformation of heterocyclic N-oxides to 2-alkyl-, aryl-, and alkenyl-substituted N-heterocycles is described. The success of this broad-scope methodology hinges on the combination of copper catalysis and activation by lithium fluoride or magnesium chloride. The utility of this method
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.