120812
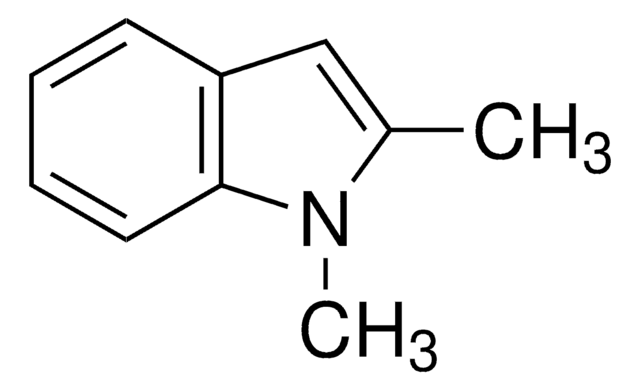
2,3-Dimethylindole
≥97%
Sinonimo/i:
NSC 24936
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C10H11N
Numero CAS:
Peso molecolare:
145.20
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
≥97%
Forma fisica
solid
P. eboll.
285 °C (lit.)
Punto di fusione
105-107 °C (lit.)
Stringa SMILE
Cc1[nH]c2ccccc2c1C
InChI
1S/C10H11N/c1-7-8(2)11-10-6-4-3-5-9(7)10/h3-6,11H,1-2H3
PYFVEIDRTLBMHG-UHFFFAOYSA-N
Applicazioni
2,3-Dimethylindole has been used to study the mechanism of oxidation of 2,3-dimethylindole by peroxodisulphate and peroxomonosulphate anions to 2-methylindole-2-carbaldehyde. It has been used to study the behaviour of methylindoles in the agilent multimode ion source by atmospheric pressure chemical ionization mass spectrometry.
Reactant for preparation of:
Reactant for:
- Bis(indolyl)methane derivatives
- Potent opioid receptor agonists
- Photorefractive materials
- Prodrugs of the cyclin-dependent kinase (CDK) inhibitor Alsterpaullone
- Dopamine receptors 2/4 (D2/D4) antagonists
- Useful azaspirocyclic building blocks
Reactant for:
- Baylis-Hillman reactions
- Photosensitized Diels-Alder reactions
- Photoinduced electron transfer reactions
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Direct evidence on the mechanism of the oxidation of 2, 3-dimethylindole by inorganic peroxo anions.
Balon M, et al.
The Journal of Organic Chemistry, 58(26), 7469-7473 (1993)
Formation of the Ions of Methylindoles in APCI Mass Spectrometry.
Liu DQ and Sun M.
ISRN Spectroscopy, 2012 (2012)
A I Novaira et al.
Journal of photochemistry and photobiology. B, Biology, 60(1), 25-31 (2001-06-02)
The quenching of anthracene fluorescence by indole, 1,2-dimethylindole (DMI), tryptophan (Trp) and indole 3-acetic acid (IAA) in palmitoyloleoylphosphatidylcholine (POPC) lipid bilayers was investigated. A very efficient quenching of the anthracene fluorescence in the lipid membrane is observed. Stern-Volmer plots are
Zhengyin Yan et al.
Analytical chemistry, 80(16), 6410-6422 (2008-07-23)
Constant neutral loss (CNL) and precursor ion (PI) scan have been widely used for the in vitro screening of glutathione conjugates derived from reactive metabolites, but these two methods are only applicable to triple quadrupole or hybrid triple quadrupole mass
Resolution of heterogeneous fluorescence from proteins and aromatic amino acids by phase-sensitive detection of fluorescence.
J R Lakowicz et al.
The Journal of biological chemistry, 256(12), 6348-6353 (1981-06-25)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.