推荐产品
方案
≥99%
mp
172-173 °C (lit.)
储存温度
2-8°C
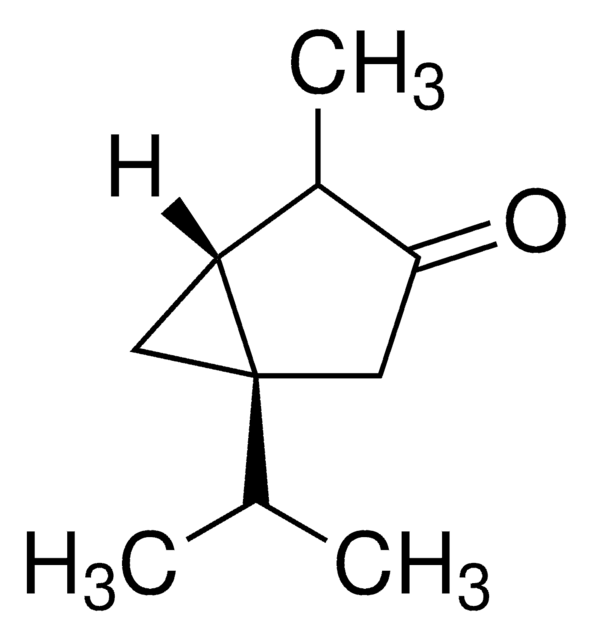
SMILES字符串
C[C@H]1[C@@H]2CC[C@@]3(C)C=CC(=O)C(C)=C3[C@H]2OC1=O
InChI
1S/C15H18O3/c1-8-10-4-6-15(3)7-5-11(16)9(2)12(15)13(10)18-14(8)17/h5,7-8,10,13H,4,6H2,1-3H3/t8-,10-,13-,15-/m0/s1
InChI key
XJHDMGJURBVLLE-BOCCBSBMSA-N
正在寻找类似产品? 访问 产品对比指南
一般描述
(−)-α-山道年是(Santonin)一种倍半萜烯内酯。它存在于青蒿属植物中。
应用
(−)-α-山道年已被用作一种桉叶烷型倍半萜烯,用于研究其对231MFP乳腺癌细胞存活率下降的影响。
生化/生理作用
(−)-α-山道年具有抗蠕虫特性。它对肠道蛔虫有治疗作用。
警示用语:
Danger
危险分类
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral
储存分类代码
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
个人防护装备
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
其他客户在看
Chapter 3 - Natural Products
Sharma S and Anand N
Pharmacognosy Journal , 25(7), 71-123 (1997)
Fungal hydroxylation of (-)-alpha-santonin
Bustos D A
Report Intl Narcotics Control Brd 03, 2, 1-6 (2012)
Parthenolide Covalently targets and inhibits focal adhesion kinase in breast cancer cells
Berdan C A, et al.
Cell Chemical Biology, 26(7), 1027-1035 (2019)
Takao Yamaura
Yakugaku zasshi : Journal of the Pharmaceutical Society of Japan, 131(3), 395-400 (2011-03-05)
Soon after its foundation in 1919, Nippon Shinyaku Co., Ltd began to develop the domestic production of Santonin, an anthelmintic agent, which, until then, had been totally imported from Russia. In 1927, Artemisia maritima ssp. monogyna was introduced from Europe
Xing Chen et al.
The journal of physical chemistry. A, 115(26), 7815-7822 (2011-06-02)
The CASSCF and CASPT2 methodologies have been used to explore the potential energy surfaces of lumisantonin in the ground and low-lying triplet states along the photoisomerization pathways. Calculations indicate that the (1)(nπ*) state is the accessible low-lying singlet state with
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持