推荐产品
生物源
synthetic (organic)
無菌
non-sterile
化驗
≥98% (HPLC)
形狀
powder
技術
inhibition assay: suitable
mp
238-240 °C (lit.)
溶解度
chloroform: methanol (1:1): 40 mg/mL, clear, colorless to faintly yellow
運輸包裝
ambient
儲存溫度
room temp
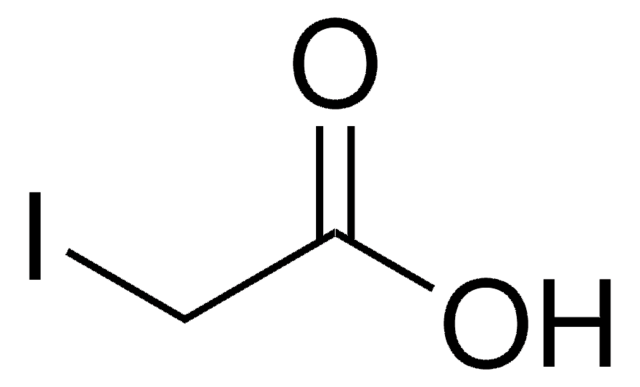
SMILES 字串
C[C@]12CC[C@H]3C(=CCc4cc(O)ccc34)[C@@H]1CCC2=O
InChI
1S/C18H20O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3-5,10,14,16,19H,2,6-9H2,1H3/t14-,16+,18+/m1/s1
InChI 密鑰
WKRLQDKEXYKHJB-HFTRVMKXSA-N
正在寻找类似产品? 访问 产品对比指南
生化/生理作用
Equilin is the Mare estrogen and is majorly used in multi-estrogen drugs for hormone replacement therapy. It is synthesized in the microsomes of mare placenta. It mediates estrogen receptor β (ERβ) expression resulting in the activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) which favors monocytes adhesion in atherosclerosis. In vitro equilin inhibits 17β-hydroxysteroid dehydrogenase type 1 (17β-HSD1) that catalyzes the conversion of estrone to 17β-estradiol.
訊號詞
Warning
危險聲明
危險分類
Carc. 2 - Lact. - Repr. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
其他客户在看
Structure of the ternary complex of human 17beta-hydroxysteroid dehydrogenase type 1 with 3-hydroxyestra-1, 3, 5, 7-tetraen-17-one (equilin) and NADP+
Sawicki MW, et al.
Proceedings of the National Academy of Sciences of the USA, 96(3), 840-845 (1999)
Equilin and equilenin biosynthesis. Stereochemistry of aromatization of 3-hydroxy-3, 5, 7-androstatrien-17-one by horse placenta
Mitsuteru N and Yoshio O
Journal of Steroid Biochemistry, 26(1), 137-143 (1987)
Equilin
Handbook of Hormones, 582-582 (2016)
Ekaterina V Rokhina et al.
The Science of the total environment, 417-418, 280-290 (2012-01-17)
Estrone (E1), 17β-estradiol (E2), estriol (E3), equilin (EQ) and 17α-estradiol (17α) estrogen hormones are released by humans and animals and have been detected in the environment and municipal wastewater treatment plants. The structural and electronic properties of natural hormone molecules
Rupinder K Bhamra et al.
Menopause (New York, N.Y.), 18(4), 393-399 (2010-11-26)
A randomized, parallel-design study was conducted to determine the pharmacokinetic profile of synthetic conjugated estrogens A (SCE-A) vaginal cream (0.625 mg SCE-A/g) when administered at intervals (1 g once daily for 7 d, then twice weekly) over a 27-day period
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门