推荐产品
形狀
solid
光學活性
[α]22/D 282.0°, c = 1 in chloroform
mp
86-94 °C
SMILES 字串
CN(C)P1Oc2ccc3CCCCc3c2-c4c(O1)ccc5CCCCc45
InChI
1S/C22H26NO2P/c1-23(2)26-24-19-13-11-15-7-3-5-9-17(15)21(19)22-18-10-6-4-8-16(18)12-14-20(22)25-26/h11-14H,3-10H2,1-2H3
InChI 密鑰
OIZQADYWBXZQKE-UHFFFAOYSA-N
應用
(11bS)-N,N-dimethyl-8,9,10,11,12,13,14,15-octahydrodinaphtho[2,1-d:1′,2′-f][1,3,2]dioxaphosphepin-4-amine is a monodentate phosphoramidite (MonoPhos) chiral ligand that can be used:
- In the Cu-catalyzed synthesis of chiral 3-(arylbut-1-yn-1-yl)silane derivatives by reacting N-tosylhydrazones with silyl-substituted alkynes via asymmetric insertion.
- In the Rh-catalyzed asymmetric hydrogenation of R-isopropylcinnamic acid derivative, a key intermediate of renin inhibitor aliskiren.
- In the Rh-catalyzed conjugate addition of arylboronic acids to lactones, enones, and nitroalkenes.
法律資訊
经 DSM 授权销售,仅供研究之用。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
A mixed-ligand approach enables the asymmetric hydrogenation of an α-isopropylcinnamic acid en route to the renin inhibitor aliskiren
Boogers JAF, et al.
Organic Process Research & Development, 11(3), 585-591 (2007)
Asymmetric Copper-Catalyzed C (sp)-H Bond Insertion of Carbenoids Derived from N-Tosylhydrazones
Osako T, et al.
Synlett, 29(17), 2251-2256 (2018)
Rhodium-catalyzed asymmetric conjugate additions of boronic acids using monodentate phosphoramidite ligands
Boiteau J, et al.
Organic Letters, 5(5), 681-684 (2003)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门![(11bR)-2,6-双(二苯基膦)-N,N-二甲基二萘并[2,1-d:1′,2′-f]-1,3,2-二氧杂膦-4-胺](/deepweb/assets/sigmaaldrich/product/structures/260/755/3101c3e8-e884-4803-ba52-a87c7e168847/640/3101c3e8-e884-4803-ba52-a87c7e168847.png)
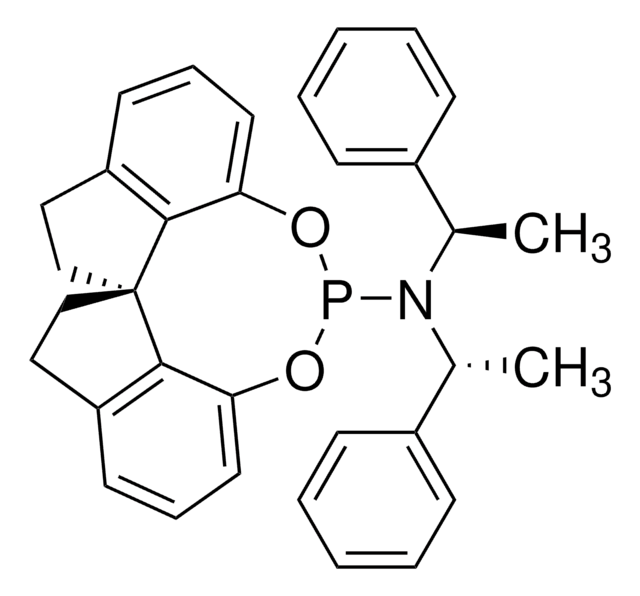
![(S,S,S)-(+)-(3,5-二氧杂-4-磷杂环庚二烯并[2,1-a:3,4-a']二萘-4-基)二(1-苯基乙基)胺 97%](/deepweb/assets/sigmaaldrich/product/structures/223/794/16c37a96-da16-488a-b3e8-7d89c47f71ee/640/16c37a96-da16-488a-b3e8-7d89c47f71ee.png)
![(S)-(+)-(3,5-二氧-4-磷-环庚并[2,1-a;3,4- a′]二萘-4-基)二甲胺 97%](/deepweb/assets/sigmaaldrich/product/structures/400/008/628143de-3954-440a-ba9c-4c0ff8e44663/640/628143de-3954-440a-ba9c-4c0ff8e44663.png)
![(S,R,R)-(+)-(3,5-二氧杂-4-磷杂环庚二烯并[2,1-a:3,4-a′]二萘-4-基)二(1-苯基乙基)胺 95% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/366/790/7555ef31-5d0b-45c9-ad40-5dfd0fe85125/640/7555ef31-5d0b-45c9-ad40-5dfd0fe85125.png)