所有图片(1)
About This Item
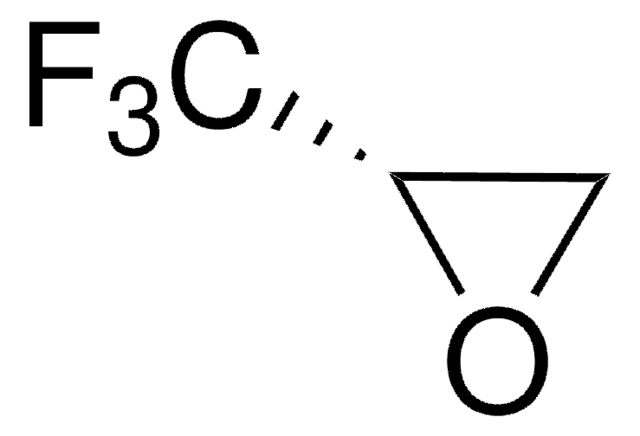
经验公式(希尔记法):
C3H3F3O
CAS号:
分子量:
112.05
MDL號碼:
分類程式碼代碼:
12352005
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
97%
折射率
n20/D <1.300
bp
25-32 °C
密度
1.294 g/mL at 25 °C
儲存溫度
2-8°C
SMILES 字串
FC(F)(F)[C@H]1CO1
InChI
1S/C3H3F3O/c4-3(5,6)2-1-7-2/h2H,1H2/t2-/m1/s1
InChI 密鑰
AQZRARFZZMGLHL-UWTATZPHSA-N
應用
(R)-(+)-3,3,3-Trifluoro-1,2-epoxypropane can be used as a substrate to synthesize:
- Substituted trifluoro amino propanols, which are found to be potent inhibitors of cholesteryl ester transfer protein.
- (2R) Trifluoro-(methoxybenzyloxy)-propanol (chiral glycol) by reacting with 4-methoxybenzyl alcohol in the presence of NaH. Chiral glycol intermediate is further utilized for the preparation of trifluoromethyl glycol carbamates as potential monoacylglycerol lipase (MAGL) inhibitors.
訊號詞
Danger
危險聲明
危險分類
Flam. Liq. 1
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
-14.8 °F
閃點(°C)
-26 °C
個人防護裝備
Eyeshields, Faceshields, Gloves
Discovery of trifluoromethyl glycol carbamates as potent and selective covalent monoacylglycerol lipase (MAGL) inhibitors for treatment of neuroinflammation
McAllister LA, et al.
Journal of Medicinal Chemistry, 61(7), 3008-3026 (2018)
Discovery of a simple picomolar inhibitor of cholesteryl ester transfer protein
Reinhard EJ, et al.
Journal of Medicinal Chemistry, 46(11), 2152-2168 (2003)
Emily J Reinhard et al.
Journal of medicinal chemistry, 46(11), 2152-2168 (2003-05-16)
A novel series of substituted N-[3-(1,1,2,2-tetrafluoroethoxy)benzyl]-N-(3-phenoxyphenyl)-trifluoro-3-amino-2-propanols is described which potently and reversibly inhibit cholesteryl ester transfer protein (CETP). Starting from the initial lead 1, various substituents were introduced into the 3-phenoxyaniline group to optimize the relative activity for inhibition of
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门