所有图片(1)
About This Item
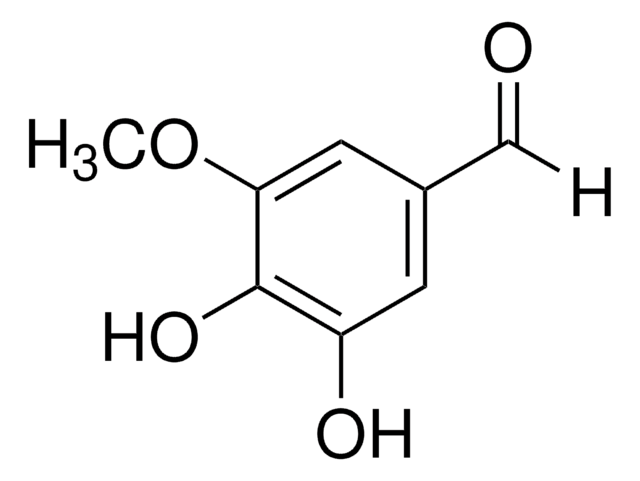
线性分子式:
(HO)3C6H2CHO
CAS号:
分子量:
154.12
MDL编号:
UNSPSC代码:
12352100
PubChem化学物质编号:
NACRES:
NA.22
推荐产品
一般描述
2,4,5-Trihydroxybenzaldehyde (2,4,5-THBA) is a tri-substituted benzaldehyde that can be prepared from sesamol. Its free radical-quenching ability, antioxidant bioactivity and cytotoxicity have been assessed. 2,4,5-THBA has been identified as one of the components in the ethyl acetate extract of Beta vulgaris var. cicla seeds. The freezing point, boiling point, density and refractive index of 2,4,5-THBA are 499.65K, 510.61K, 1.3725g/cm3 and 1.6400, respectively.
应用
2,4,5-Trihydroxybenzaldehyde may be used in the synthesis of:
- 2,4,5-tribenzyloxybenzaldehyde
- 2,4,5-trihydroxybenzaldehyde N-(diphenylmethylene)hydrazine
- 2-hydroxy-4,5-dimethoxybenzaldehyde
警示用语:
Warning
危险声明
危险分类
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
靶器官
Respiratory system
储存分类代码
11 - Combustible Solids
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
个人防护装备
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Ahluwalia VK.
Intermediates for Organic Synthesis, 51-51 (2010)
T H Tseng et al.
Toxicology, 161(3), 179-187 (2001-04-12)
As part of our earlier search for new compounds with improved biological activities including antioxidant, anti-inflammatory, and tumor growth inhibition activities, we synthesized 2,4,5-trihydroxybenzaldehyde (2,4,5-THBA) from commercially available Sesamol. First we examined the free radical-quenching capacity of 2,4,5-THBA, 3,4-dihydroxybenzaldehyde (3,4-DHBA)
Yaws CL.
The Yaws Handbook of Physical Properties for Hydrocarbons and Chemicals, 146-146 (2015)
Khalid Mohammed Khan et al.
Medicinal chemistry (Shariqah (United Arab Emirates)), 9(4), 588-595 (2012-11-16)
Benzophenonehydrazone Schiff bases 1-25 were synthesized and their in vitro antiglycation potential has been studied. Out of twenty-five compounds, thirteen showed varying degrees of antiglycation activity with IC50 values ranging between 25.7 - 305 μM, if compared with the standard
Stephen J Mills et al.
Chembiochem : a European journal of chemical biology, 9(11), 1757-1766 (2008-06-25)
Novel benzene polyphosphates were synthesised as inositol polyphosphate mimics and evaluated against type-I inositol 1,4,5-trisphosphate 5-phosphatase, which only binds soluble inositol polyphosphates, and against the PH domain of protein kinase Balpha (PKBalpha), which can bind both soluble inositol polyphosphates and
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持