所有图片(1)
About This Item
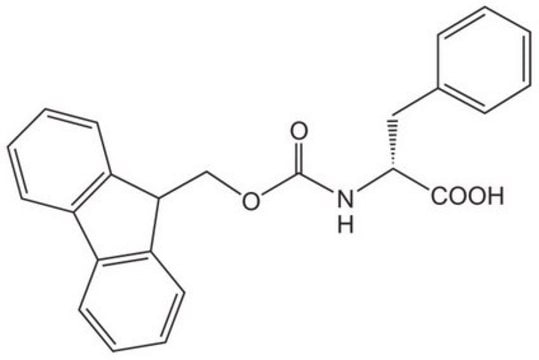
经验公式(希尔记法):
C31H30N2O6
CAS号:
分子量:
526.58
Beilstein:
7062970
MDL號碼:
分類程式碼代碼:
12352209
eCl@ss:
32160406
PubChem物質ID:
NACRES:
NA.26
推荐产品
化驗
≥95.0% (HPLC)
形狀
solid
反應適用性
reaction type: Fmoc solid-phase peptide synthesis
應用
peptide synthesis
官能基
Boc
Fmoc
儲存溫度
2-8°C
SMILES 字串
CC(C)(C)OC(=O)n1cc(C[C@@H](NC(=O)OCC2c3ccccc3-c4ccccc24)C(O)=O)c5ccccc15
InChI
1S/C31H30N2O6/c1-31(2,3)39-30(37)33-17-19(20-10-8-9-15-27(20)33)16-26(28(34)35)32-29(36)38-18-25-23-13-6-4-11-21(23)22-12-5-7-14-24(22)25/h4-15,17,25-26H,16,18H2,1-3H3,(H,32,36)(H,34,35)/t26-/m1/s1
InChI 密鑰
ADOHASQZJSJZBT-AREMUKBSSA-N
相關產品
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
Fayçal Touti et al.
Nature chemical biology, 15(4), 410-418 (2019-03-20)
The use of competitive inhibitors to disrupt protein-protein interactions (PPIs) holds great promise for the treatment of disease. However, the discovery of high-affinity inhibitors can be a challenge. Here we report a platform for improving the affinity of peptide-based PPI
Ngoc-Duc Doan et al.
Journal of peptide science : an official publication of the European Peptide Society, 21(5), 387-391 (2014-11-18)
The solid-phase synthesis of azapeptides possessing a C-terminal aza-residue has been accomplished by a protocol featuring regioselective alkylation of benzhydrylidene-aza-glycinamide and illustrated by the syntheses of [aza-Lys(6)] growth-hormone-releasing peptide-6 analogs.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门