推荐产品
质量水平
方案
97%
表单
solid
旋光性
[α]20/D −200°, c = 1 in chloroform
mp
174-176 °C (lit.)
官能团
ester
SMILES字符串
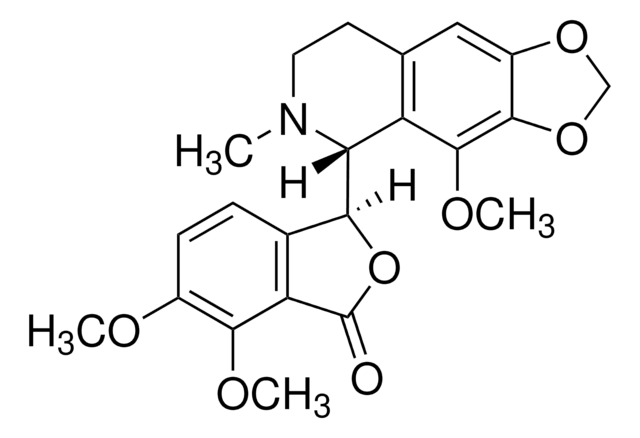
COc1ccc2C(OC(=O)c2c1OC)C3N(C)CCc4cc5OCOc5c(OC)c34
InChI
1S/C22H23NO7/c1-23-8-7-11-9-14-20(29-10-28-14)21(27-4)15(11)17(23)18-12-5-6-13(25-2)19(26-3)16(12)22(24)30-18/h5-6,9,17-18H,7-8,10H2,1-4H3/t17-,18+/m1/s1
InChI key
AKNNEGZIBPJZJG-MSOLQXFVSA-N
正在寻找类似产品? 访问 产品对比指南
一般描述
(S,R)-Noscapine is a phthalideisoquinoline alkaloid found in opium. It is an antimicrotubule agent that also shows potent antitumor activity.
应用
Noscapine may be used as a source to synthesize its bromo-derivatives, which has higher tubulin binding activity when compared to noscapine. Its 3,4,5-trimethoxybenzyl analog is a potential antitumor agent.
警示用语:
Warning
危险声明
预防措施声明
危险分类
Acute Tox. 4 Oral - STOT SE 3
靶器官
Central nervous system
储存分类代码
11 - Combustible Solids
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
个人防护装备
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
V Pannu et al.
Cell death & disease, 3, e346-e346 (2012-07-13)
Centrosome amplification (CA) and resultant chromosomal instability have long been associated with tumorigenesis. However, exacerbation of CA and relentless centrosome declustering engender robust spindle multipolarity (SM) during mitosis and may induce cell death. Recently, we demonstrated that a noscapinoid member
Zhong-Ze Fang et al.
British journal of pharmacology, 167(6), 1271-1286 (2012-06-08)
Noscapine is a promising anti-tumour agent. The purpose of the present study was to describe the metabolic map and investigate the bioactivation of noscapine. Ultra-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight mass spectrometry-based metabolomics was used to analyse
Pradeep K Naik et al.
Journal of computer-aided molecular design, 25(5), 443-454 (2011-05-06)
Noscapine and its derivatives are important microtubule-interfering agents shown to have potent anti-tumor activity. The binding free energies (ΔG (bind)) of noscapinoids computed using linear interaction energy (LIE) method with a surface generalized Born (SGB) continuum solvation model were in
Niyati Jhaveri et al.
Cancer letters, 312(2), 245-252 (2011-09-20)
Noscapine, a common oral antitussive agent, has been shown to have potent antitumor activity in a variety of cancers. Treatment of glioblastoma multiforme (GBM) with temozolomide (TMZ), its current standard of care, is problematic because the tumor generally recurs and
Thilo Winzer et al.
Science (New York, N.Y.), 336(6089), 1704-1708 (2012-06-02)
Noscapine is an antitumor alkaloid from opium poppy that binds tubulin, arrests metaphase, and induces apoptosis in dividing human cells. Elucidation of the biosynthetic pathway will enable improvement in the commercial production of noscapine and related bioactive molecules. Transcriptomic analysis
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持