所有图片(1)
About This Item
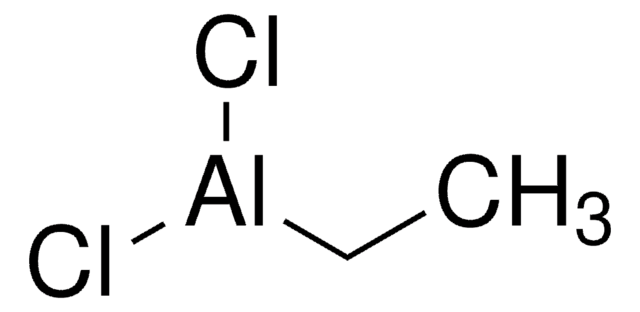
线性分子式:
CH3CH2AlCl2
CAS号:
分子量:
126.95
Beilstein:
4123357
MDL编号:
UNSPSC代码:
12352103
PubChem化学物质编号:
NACRES:
NA.22
推荐产品
应用
Ethylaluminum dichloride is a strong Lewis acid and a proton scavenger that can be used:
- To catalyze polymerization of isobutylene.
- To facilitate Diels-Alder reaction of α,β-unsaturated esters.
- To carry out Pinacol reduction rearrangements.
- To promote Schmidt rearrangement of diketoazides in the synthesis of indolizidines and pyrroloazepinediones.
警示用语:
Danger
危险分类
Aquatic Chronic 2 - Asp. Tox. 1 - Eye Dam. 1 - Flam. Liq. 2 - Repr. 2 - Skin Corr. 1B - STOT RE 1 Inhalation - STOT SE 3 - Water-react 2
靶器官
Central nervous system, Nervous system
储存分类代码
4.2 - Pyrophoric and self-heating hazardous materials
WGK
WGK 3
闪点(°F)
-9.4 °F - closed cup
闪点(°C)
-23 °C - closed cup
其他客户在看
Concise access to indolizidine and pyrroloazepine skeleta via intramolecular Schmidt reactions of azido 1, 3-diketones.
Lertpibulpanya D and Marsden S P
Organic & Biomolecular Chemistry, 4(18), 3498-3504 (2006)
Kinetic and Mechanistic Studies of the Polymerization of Isobutylene Catalyzed by EtAlCl2/Bis (2-chloroethyl) Ether Complex in Hexanes.
Banerjee S, et al.
Macromolecules, 48(16), 5474-5480 (2015)
Cyclorearrangement and cycloolefination of keto-bis-sulfones. A sulfone analog of a pinacol reduction-rearrangement.
Trost B M, et al.
Journal of the American Chemical Society, 114(13), 5432-5434 (1992)
Stereochemical aspects of the intramolecular Diels-Alder reactions of methyl deca-2, 7, 9-trienoates. 1. Thermal cyclizations.
Roush W R, et al.
The Journal of Organic Chemistry, 45(21), 4264-4267 (1980)
Ethylaluminum Dichloride.
Snider B B.
e-EROS Encyclopedia of Reagents for Organic Synthesis. (2001)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持