P2016
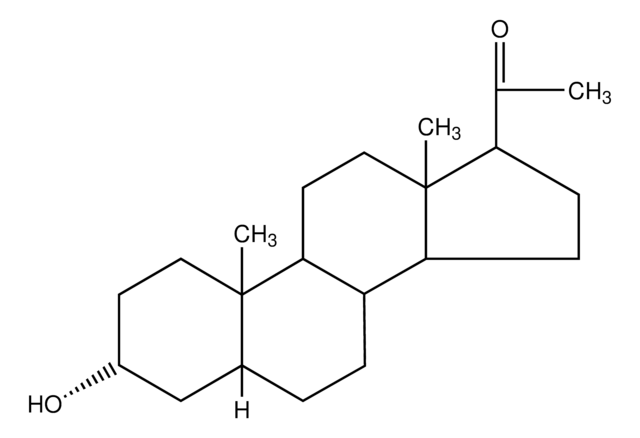
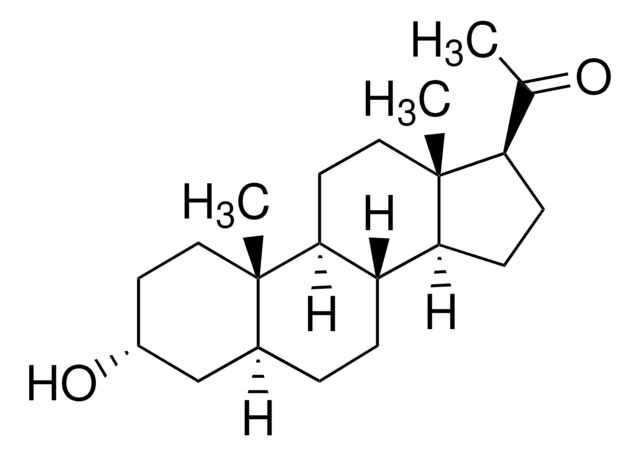
3α,21-Dihydroxy-5α-pregnan-20-one
≥95%
Sinónimos:
21-Hydroxyallopregnanolone, 5α-Pregnane-3α,21-diol-20-one, 5α-THDOC, Allopregnane-3α,21-diol-20-one, Allotetrahydrodeoxycorticosterone
Seleccione un Tamaño
282,80 €
Precio de catálogo404,00 €Ahorre 30 %Seleccione un Tamaño
About This Item
282,80 €
Precio de catálogo404,00 €Ahorre 30 %Productos recomendados
Nivel de calidad
Ensayo
≥95%
Formulario
powder
cadena SMILES
[H][C@@]12CC[C@@]3([H])[C@]4([H])CC[C@H](C(=O)CO)[C@@]4(C)CC[C@]3([H])[C@@]1(C)CC[C@@H](O)C2
InChI
1S/C21H34O3/c1-20-9-7-14(23)11-13(20)3-4-15-16-5-6-18(19(24)12-22)21(16,2)10-8-17(15)20/h13-18,22-23H,3-12H2,1-2H3/t13-,14+,15-,16-,17-,18+,20-,21-/m0/s1
Clave InChI
CYKYBWRSLLXBOW-GDYGHMJCSA-N
Información sobre el gen
human ... GABRA1(2554) , GABRA2(2555) , GABRA3(2556) , GABRA4(2557) , GABRA5(2558) , GABRA6(2559) , GABRB1(2560) , GABRB2(2561) , GABRB3(2562)
rat ... Gabra2(29706)
Descripción general
Aplicación
- as an γ-aminobutyric acid type A receptor (γ-GABAA) agonist in voltage-clamp measurements studies in Xenopus oocytes[2]
- to test its effect on the spike-wave discharges (SWD) finasteride-treated rats[3]
- in electrophysiological studies with dentate granule cells (DGCs) post controlled cortical impact (CCI) -induced brain injury[4]
Acciones bioquímicas o fisiológicas
Características y beneficios
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Artículos
DISCOVER Bioactive Small Molecules for Neuroscience
Filtros activos
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico