E5630
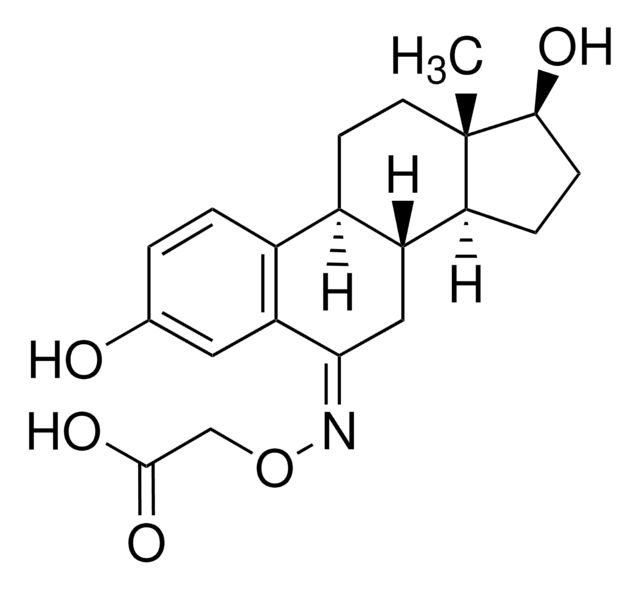
β-Estradiol 6-(O-carboxymethyl)oxime: BSA
About This Item
Productos recomendados
esterilidad
non-sterile
Nivel de calidad
formulario
lyophilized powder
Extensión del etiquetado
~30 mol steroid per mol BSA
solubilidad
phosphate buffer: 0.90-1.10 mg/mL, faintly hazy to hazy, colorless to faintly yellow
Condiciones de envío
ambient
temp. de almacenamiento
2-8°C
Categorías relacionadas
Descripción general
Aplicación
- to test its effect on the N-methyl-D-aspartate receptor (NMDAR) currents in female dorsal root ganglion (DRG) neurons
- to coat microplates for the detection of plasma 17β-Estradiol levels using indirect enzyme-linked immunosorbent assay (ELISA)
- to test its effect on MutL homolog 1 in colorectal cancer lines
Acciones bioquímicas o fisiológicas
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Carc. 2
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico