C2196
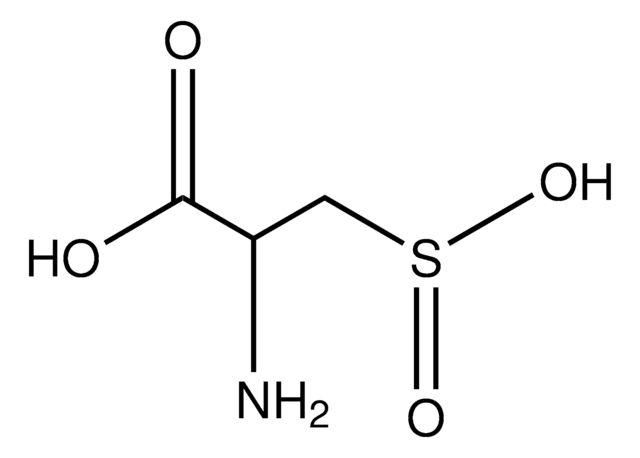
L-Cysteine S-sulfate
≥98% (TLC), suitable for ligand binding assays
Synonyma:
S-Sulfo-L-cysteine
About This Item
Doporučené produkty
product name
L-Cysteine S-sulfate, ≥98% (TLC)
Quality Level
assay
≥98% (TLC)
form
powder
technique(s)
ligand binding assay: suitable
color
white
storage temp.
−20°C
SMILES string
N[C@@H](CSS(O)(=O)=O)C(O)=O
InChI
1S/C3H7NO5S2/c4-2(3(5)6)1-10-11(7,8)9/h2H,1,4H2,(H,5,6)(H,7,8,9)/t2-/m0/s1
InChI key
NOKPBJYHPHHWAN-REOHCLBHSA-N
Hledáte podobné produkty? Navštivte Průvodce porovnáváním produktů
Biochem/physiol Actions
Cysteine is one of the functional amino acids that are associated with growth, reproduction, maintenance and immunity. Cysteine is a source of disulfide linkage in protein and is associated with sulfur transport. At physiological pH, cysteine undergoes rapid oxidation to form cystine. Reduced availability of cysteine or cystine, influences leukocyte metabolism. L-Cysteine serves as a precursor for the rate limiting step in glutathione synthesis that occurs in neurons. It donates inorganic sulfate for detoxification reactions. Therefore, L-cysteine might be associated with neuroprotection. It is found to obstruct the entry of heavy metal ions across the blood-brain barrier into the brain. Increased levels of L-cysteine might lead to neurotoxicity.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Osvědčení o analýze (COA)
Vyhledejte osvědčení Osvědčení o analýze (COA) zadáním čísla šarže/dávky těchto produktů. Čísla šarže a dávky lze nalézt na štítku produktu za slovy „Lot“ nebo „Batch“.
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
Zákazníci si také prohlíželi
Náš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.