471313
Tributylamine
≥98.5%
Synonyma:
Tri-n-butylamine
About This Item
Doporučené produkty
vapor density
6.38 (vs air)
Quality Level
vapor pressure
0.3 mmHg ( 20 °C)
2.4 mmHg ( 55 °C)
assay
≥98.5%
form
liquid
autoignition temp.
410 °F
expl. lim.
6 %
refractive index
n20/D 1.428 (lit.)
pH
10.2 (25 °C, 0.1 g/L)
bp
216 °C (lit.)
mp
−70 °C (lit.)
density
0.778 g/mL at 25 °C (lit.)
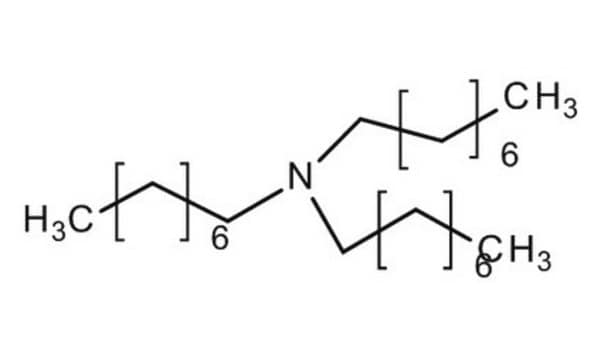
SMILES string
CCCCN(CCCC)CCCC
InChI
1S/C12H27N/c1-4-7-10-13(11-8-5-2)12-9-6-3/h4-12H2,1-3H3
InChI key
IMFACGCPASFAPR-UHFFFAOYSA-N
Hledáte podobné produkty? Navštivte Průvodce porovnáváním produktů
General description
Application
- An extraction solvent with CHCA (α-cyano-4-hydroxycinnamic acid) for the selective phospholipids (PLs) extraction from EVOO (extra virgin olive oil) and HO (hazelnut oil).
- A hydroxylating agent in the synthesis of spinel nickel ferrites (NiFe2O4) nanoparticles (NPs).
Features and Benefits
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 1 Inhalation - Acute Tox. 2 Dermal - Acute Tox. 4 Oral - Skin Irrit. 2
Storage Class
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
wgk_germany
WGK 1
flash_point_f
145.4 °F - closed cup
flash_point_c
63 °C - closed cup
Osvědčení o analýze (COA)
Vyhledejte osvědčení Osvědčení o analýze (COA) zadáním čísla šarže/dávky těchto produktů. Čísla šarže a dávky lze nalézt na štítku produktu za slovy „Lot“ nebo „Batch“.
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
Zákazníci si také prohlíželi
Náš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.