W239607
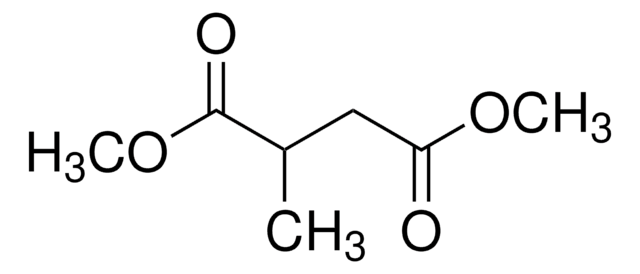
Dimethyl succinate
98%, FG
Synonyma:
Dimethyl butanedioate, Succinic acid dimethyl ester
About This Item
Halal
Kosher
Doporučené produkty
biological source
synthetic
Quality Level
grade
FG
Halal
Kosher
reg. compliance
EU Regulation 1334/2008 & 178/2002
FDA 21 CFR 117
FDA 21 CFR 172.515
vapor pressure
0.3 mmHg ( 20 °C)
assay
98%
autoignition temp.
689 °F
expl. lim.
8.5 %
refractive index
n20/D 1.419 (lit.)
bp
200 °C (lit.)
mp
16-19 °C (lit.)
density
1.117 g/mL at 25 °C (lit.)
application(s)
flavors and fragrances
documentation
see Safety & Documentation for available documents
food allergen
no known allergens
organoleptic
green; fruity; floral; sweet
SMILES string
COC(=O)CCC(=O)OC
InChI
1S/C6H10O4/c1-9-5(7)3-4-6(8)10-2/h3-4H2,1-2H3
InChI key
MUXOBHXGJLMRAB-UHFFFAOYSA-N
Hledáte podobné produkty? Navštivte Průvodce porovnáváním produktů
General description
Application
- Metabolic Bypass Rescues Aberrant S-nitrosylation-Induced TCA Cycle Inhibition and Synapse Loss in Alzheimer′s Disease Human Neurons.: This research discusses the role of metabolic intermediates like Dimethyl succinate in bypassing biochemical blocks in Alzheimer′s disease, offering potential therapeutic avenues (Andreyev et al., 2024).
- Update to RIFM fragrance ingredient safety assessment, dimethyl succinate, CAS Registry Number 106-65-0.: A comprehensive safety assessment of Dimethyl succinate as a fragrance ingredient, highlighting its toxicological profile and safe usage parameters (Api et al., 2023).
signalword
Warning
hcodes
pcodes
Hazard Classifications
Eye Irrit. 2
Storage Class
10 - Combustible liquids
wgk_germany
WGK 1
flash_point_f
201.2 °F - closed cup
flash_point_c
94 °C - closed cup
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Vyberte jednu z posledních verzí:
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
Zákazníci si také prohlíželi
Náš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.