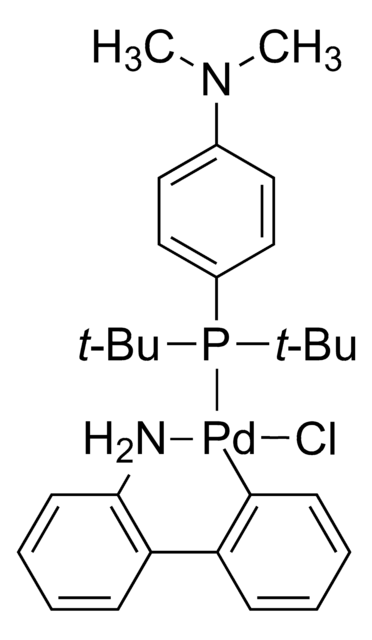
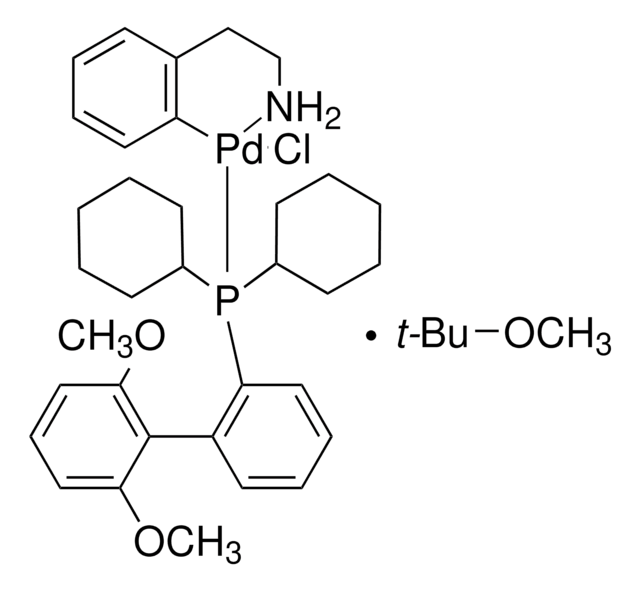
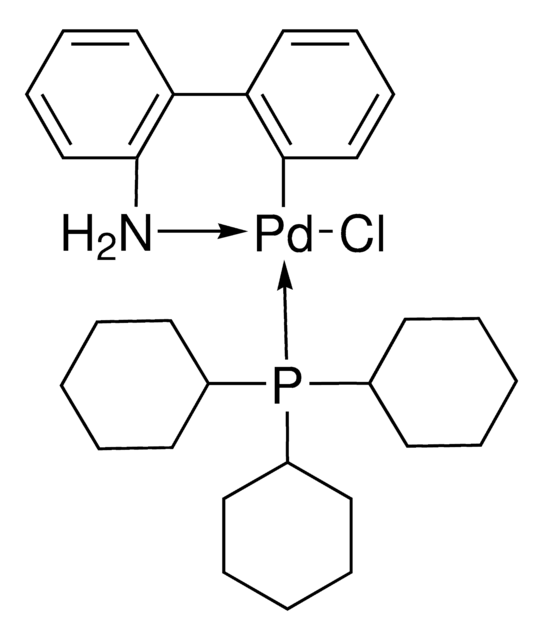
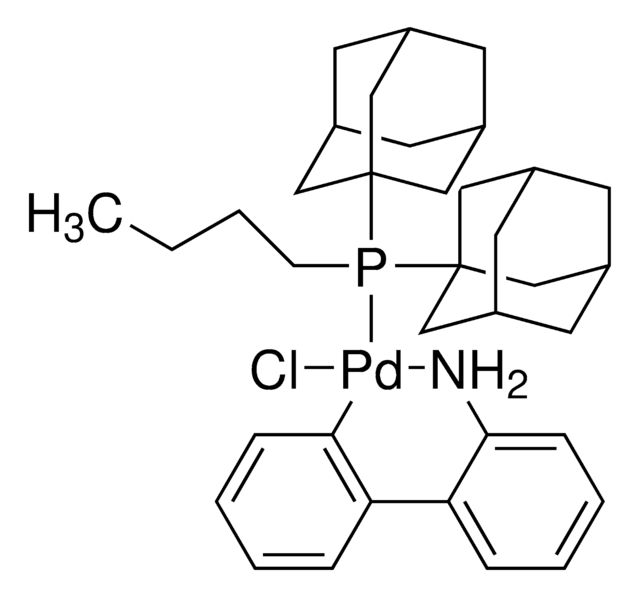
741825
XPhos Pd G2
98%
Synonyma:
2nd Generation XPhos Precatalyst, Chloro(2-dicyclohexylphosphino-2′,4′,6′-triisopropyl-1,1′-biphenyl)[2-(2′-amino-1,1′-biphenyl)]palladium(II), X-Phos aminobiphenyl palladium chloride precatalyst, XPhos-Pd-G2
About This Item
Doporučené produkty
Quality Level
assay
98%
form
solid
feature
generation 2
reaction suitability
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
reaction type: Cross Couplings
mp
202-210 °C
functional group
phosphine
SMILES string
Nc1ccccc1-c2ccccc2[Pd]Cl.Nc3ccccc3-c4ccccc4[Pd]Cl.CC(C)c5cc(C(C)C)c(-c6cccc(c6)P(C7CCCCC7)C8CCCCC8)c(c5)C(C)C.CC(C)c9cc(C(C)C)c(c(c9)C(C)C)-c%10ccccc%10P(C%11CCCCC%11)C%12CCCCC%12
InChI
1S/2C33H49P.2C12H10N.2ClH.2Pd/c1-23(2)27-21-31(24(3)4)33(32(22-27)25(5)6)26-14-13-19-30(20-26)34(28-15-9-7-10-16-28)29-17-11-8-12-18-29;1-23(2)26-21-30(24(3)4)33(31(22-26)25(5)6)29-19-13-14-20-32(29)34(27-15-9-7-10-16-27)28-17-11-8-12-18-28;2*13-12-9-5-4-8-11(12)10-6-2-1-3-7-10;;;;/h13-14,19-25,28-29H,7-12,15-18H2,1-6H3;13-14,19-25,27-28H,7-12,15-18H2,1-6H3;2*1-6,8-9H,13H2;2*1H;;/q;;;;;;2*+1/p-2
InChI key
HMRJFNBZAWHTGN-UHFFFAOYSA-L
General description
Application
- Palladium-catalyzed Suzuki-Miyaura coupling reactions of potassium organotrifluoroborates and sulfamates.
- Suzuki-Miyaura cross-coupling reactions of sensitive aryl and heteroarylboronic acids.
- Synthesis of potassium Boc-protected secondary aminomethyltrifluoroborates, via Suzuki-Miyaura cross-coupling reaction.
related product
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Vyberte jednu z posledních verzí:
Osvědčení o analýze (COA)
Nevidíte správnou verzi?
Potřebujete-li konkrétní verzi, můžete vyhledat daný certifikát podle čísla dávky nebo čísla šarže.
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
Zákazníci si také prohlíželi
Sortimentní položky
Buchwald precatalysts and ligands are bulky electron-rich dialkylbiaryl phospine-based catalysts and structurally related ligands that improve reactivity in Pd-catalyzed cross - coupling reactions
Související obsah
Explore reliable, premium grade catalysis materials for your pharma or industrial project. Specialty chemicals and formulations are available in bulk quantities and volumes from a few grams to multi-metric tons with complete documentation to simplify your leap from development to commercialization.
Náš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.


![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)