706531
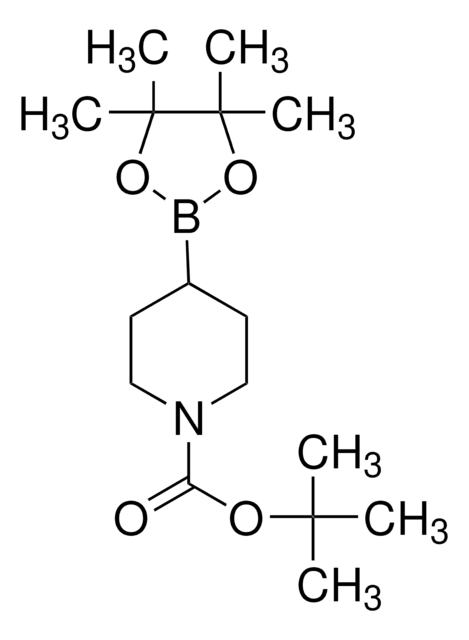
N-Boc-1,2,3,6-tetrahydropyridine-4-boronic acid pinacol ester
95%
Synonyma:
(1-tert-Butoxycarbonyl-1,2,3,6-tetrahydropyridin-4-yl)boronic acid pinacol ester, (N-tert-Butoxycarbonyl-1,2,3,6-tetrahydropyridin-4-yl)boronic acid pinacol ester, (N-tert-Butoxycarbonyl-1,2,5,6-tetrahydropyridin-4-yl)boronic acid pinacol ester, (N-tert-Butoxycarbonyl)-1,2,3,6-tetrahydropyridine-4-boronic acid pinacol ester
About This Item
Doporučené produkty
assay
95%
form
powder
mp
100-114 °C
SMILES string
CC(C)(C)OC(=O)N1CCC(=CC1)B2OC(C)(C)C(C)(C)O2
InChI
1S/C16H28BNO4/c1-14(2,3)20-13(19)18-10-8-12(9-11-18)17-21-15(4,5)16(6,7)22-17/h8H,9-11H2,1-7H3
InChI key
VVDCRJGWILREQH-UHFFFAOYSA-N
Související kategorie
General description
Application
- Suzuki-Miyaura cross-coupling using palladium phosphine catalyst
- Palladium-catalyzed ligand-controlled regioselective Suzuki coupling
- Palladium-catalyzed Suzuki-Miyaura coupling
- Suzuki coupling followed by iodolactonization reaction
- Wrenchnolol derivative optimized for gene activation in cells
Reagent used in Preparation of several enzymatic inhibitors and receptor ligands
- Orally active anaplastic lymphoma kinase inhibitors
- Oxazolecarboxamides as diacylglycerol acyltransferase-1 inhibitors for treatment of obesity and diabetes
- 4-arylpiperidinyl amides and N-arylpiperidin-3-yl-cyclopropanecarboxamides as novel melatonin receptor ligands
- Quinazoline analogs as glucocerebrosidase inhibitors with chaperone activity for treatment of Gaucher disease, a lysosomal storage disorder
- Arylpiperazine and piperidine ethers as dual acting norepinephrine reuptake inhibitors and 5-HT1A partial agonists
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Osvědčení o analýze (COA)
Vyhledejte osvědčení Osvědčení o analýze (COA) zadáním čísla šarže/dávky těchto produktů. Čísla šarže a dávky lze nalézt na štítku produktu za slovy „Lot“ nebo „Batch“.
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
Zákazníci si také prohlíželi
Náš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.





![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)

