54340
2-Hydroxyethylhydrazine
≥95% (GC)
Synonyma:
2-Hydrazinoethanol
About This Item
Doporučené produkty
assay
≥95% (GC)
refractive index
n20/D 1.493 (lit.)
n20/D 1.493
bp
155-160 °C/32 mmHg (lit.)
mp
−70 °C (lit.)
density
1.123 g/mL at 25 °C (lit.)
functional group
amine
hydrazine
hydroxyl
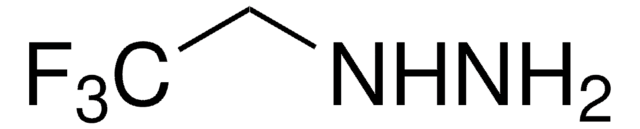
SMILES string
NNCCO
InChI
1S/C2H8N2O/c3-4-1-2-5/h4-5H,1-3H2
InChI key
GBHCABUWWQUMAJ-UHFFFAOYSA-N
Hledáte podobné produkty? Navštivte Průvodce porovnáváním produktů
General description
Other Notes
signalword
Danger
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Carc. 2 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
flash_point_f
170.6 °F - closed cup
flash_point_c
77 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Vyberte jednu z posledních verzí:
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
Náš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.