47714
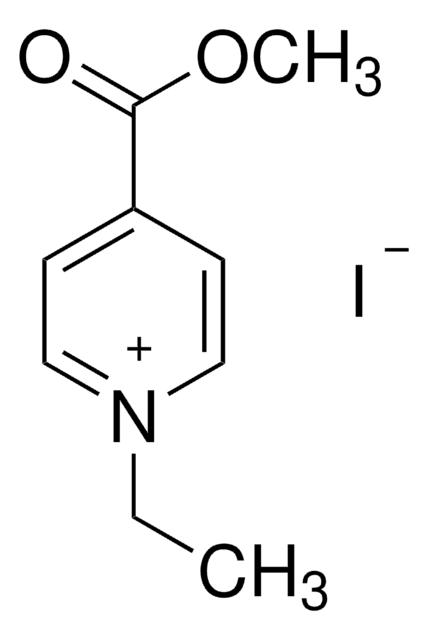
4-Formyl-1-methylpyridinium benzenesulfonate
≥95.0%
Přihlásitk zobrazení cen stanovených pro organizaci a smluvních cen
About This Item
Empirický vzorec (Hillův zápis):
C13H13NO4S
Číslo CAS:
Molekulová hmotnost:
279.31
Beilstein/REAXYS Number:
5695963
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Doporučené produkty
Quality Level
assay
≥95.0%
form
solid
impurities
≤2.0% water
mp
~95 °C
functional group
aldehyde
sulfonic acid
SMILES string
[H]C(=O)c1cc[n+](C)cc1.[O-]S(=O)(=O)c2ccccc2
InChI
1S/C7H8NO.C6H6O3S/c1-8-4-2-7(6-9)3-5-8;7-10(8,9)6-4-2-1-3-5-6/h2-6H,1H3;1-5H,(H,7,8,9)/q+1;/p-1
InChI key
HSVLGIFAXFDLMU-UHFFFAOYSA-M
Hledáte podobné produkty? Navštivte Průvodce porovnáváním produktů
General description
4-Formyl-1-methylpyridinium benzenesulfonate is a pyridinium salt widely used for the conversion of primary amines to the carbonyl compounds like aldehydes and ketones. The reaction conditions are mild, suitable for compounds with sensitive functional groups thereby providing an efficient alternative for such transformations.
Application
4-Formyl-1-methylpyridinium benzenesulfonate may be used as a reagent in the synthesis of the following:
- tetrazolic analogs of chalcones
- (+)-ferruginol
- Ecteinascidin 743
- Galipea alkaloids
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Vyberte jednu z posledních verzí:
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
Zacharias Amara et al.
Natural product reports, 30(9), 1211-1225 (2013-07-31)
This review focuses on recent applications of the aza-Michael reaction in alkaloids total synthesis with a special emphasis on stereoselectivity. The report highlights achievements and challenges over the past five years and describes stereoselective intra- and inter-molecular conjugate addition of
Jinchun Chen et al.
Journal of the American Chemical Society, 128(1), 87-89 (2006-01-05)
A convergent total synthesis of ecteinascidin 743 is realized from five building blocks of almost equal size. It takes 23 steps from l-3-hydroxy-4-methoxy-5-methyl phenylalanol (5) with an overall yield of 3%.
Short syntheses of (+)-ferruginol from (+)-dehydroabietylamine.
Gonzalez MA and Perez-Guaita D.
Tetrahedron, 68(47), 9612-9615 (2012)
Ornella Mesenzani et al.
Bioorganic & medicinal chemistry letters, 21(2), 764-768 (2010-12-21)
In the chalcone scaffold, it is thought that the double bond is an important structural linker but it is likely not essential for the interaction with tubulin. Yet, it may be a potential site of metabolic degradation and interaction with
Mild and simple biomimetic conversion of amines to carbonyl compounds.
Buckley TF and Rapoport H
Journal of the American Chemical Society, 104(16), 4446-4450 (1982)
Náš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.