432725
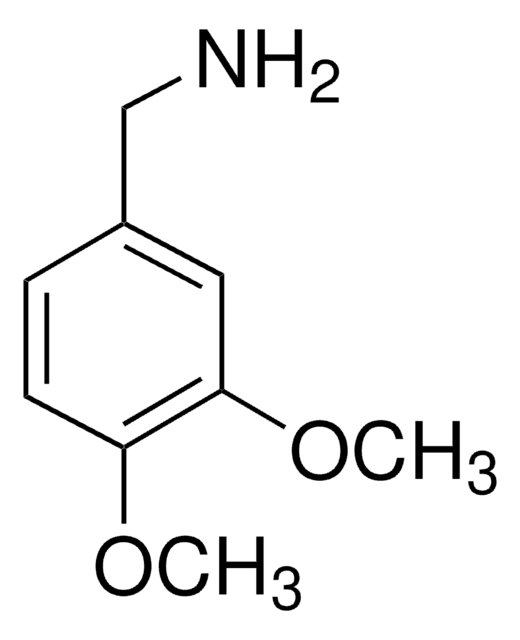
2,4-Dimethoxybenzylamine
98%
Synonyma:
(2,4-Dimethoxyphenyl)methanamine, 1-(2,4-Dimethoxyphenyl)methanamine, 2,4-Dimethoxybenzenemethanamine, 2,4-Dimethyloxybenzylamine, [(2,4-Dimethoxyphenyl)methyl]amine
About This Item
Doporučené produkty
assay
98%
refractive index
n20/D 1.549 (lit.)
bp
140 °C/1 mmHg (lit.)
density
1.113 g/mL at 25 °C (lit.)
functional group
amine
SMILES string
COc1ccc(CN)c(OC)c1
InChI
1S/C9H13NO2/c1-11-8-4-3-7(6-10)9(5-8)12-2/h3-5H,6,10H2,1-2H3
InChI key
QOWBXWFYRXSBAS-UHFFFAOYSA-N
General description
Application
- As an ammonia equivalent in the concise synthesis of a series of 2,4,5-trisubstituted oxazoles, via a tandem Ugi/Robinson-Gabriel reaction sequence.
- Total synthesis of (-)-muraymycin (MRY) D2 and its epimer, the antibacterial nucleoside natural product.
- Two-step synthesis of amide derivatives of uracil polyoxin C (UPOC) methyl ester using the Ugi reaction.
- Synthesis of N-hydroxythiourea.
- Synthesis of anti-HIV-1 agents.
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 3
flash_point_f
235.4 °F - closed cup
flash_point_c
113 °C - closed cup
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Vyberte jednu z posledních verzí:
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
Zákazníci si také prohlíželi
Náš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.