PHR1669
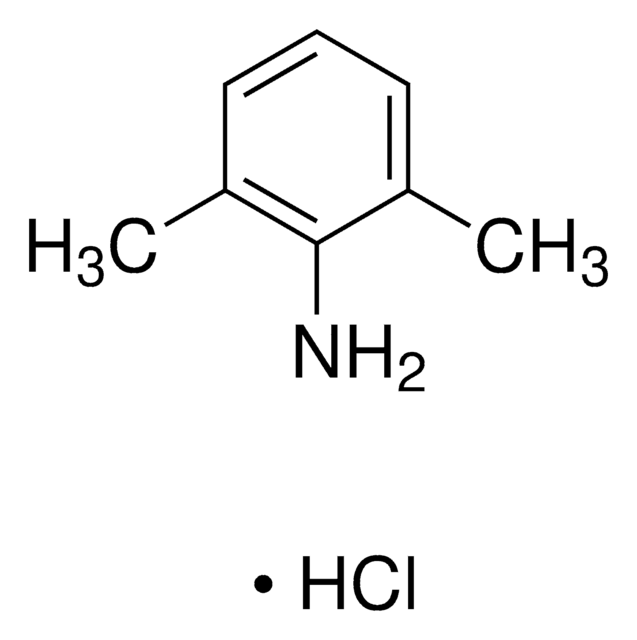
2,6-Dimethylaniline
Pharmaceutical Secondary Standard; Certified Reference Material
Sinónimos:
2,6-Dimethylaniline, Lidocaine Impurity A; 2,6 DMA, 2,6-Xylidine, 2-Amino-1,3-dimethylbenzene, 2-Amino-m-xylene
About This Item
Productos recomendados
grade
certified reference material
pharmaceutical secondary standard
Quality Level
agency
traceable to Ph. Eur. Y0001575
vapor pressure
<0.01 mmHg ( 20 °C)
API family
lidocaine
CofA
current certificate can be downloaded
packaging
pkg of 100 mg
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
refractive index
n20/D 1.560 (lit.)
bp
214 °C/739 mmHg (lit.)
mp
10-12 °C (lit.)
density
0.984 g/mL at 25 °C (lit.)
application(s)
pharmaceutical (small molecule)
format
neat
storage temp.
2-30°C
SMILES string
Cc1cccc(C)c1N
InChI
1S/C8H11N/c1-6-4-3-5-7(2)8(6)9/h3-5H,9H2,1-2H3
InChI key
UFFBMTHBGFGIHF-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
The standard is a certified reference material (CRM) qualified with instruments validated according to good manufacturing practices (GMP) using pharmacopeia monograph methods. It is supplied with a comprehensive certificate containing information on traceability assay results, certified purity, homogeneity tests, uncertainty statement, and stability assessment.
Lidocaine Related Compound A is a primary aromatic amine and a major metabolite of the anesthetic lidocaine. It is used as a starting material in the manufacturing of various anesthetics like lidocaine, bupivacaine, mepivacaine, etidocaine, ropivacaine, pyrrocaine, and xylazine.
Application
This pharmaceutical secondary standard can also be used as follows:
- Development of an impurity selective reverse phase-high performance liquid chromatography (RP-HPLC) method to determine dexpanthenol, lidocaine hydrochloride, mepyramine maleate, and their related substances in topical dosage forms
- Testing a selective high-performance liquid chromatography-diode array detection (HPLC-DAD) method, developed for the simultaneous analysis of miconazole nitrate and lidocaine hydrochloride in their combined oral gel dosage form, for its stability-indicating properties
- Evaluation of a high-performance liquid chromatography-diode array detection (HPLC-DAD) procedure― for its stability indicating properties, developed to determine nitrofurazone and lidocaine hydrochloride in their combined dosage form
- Separation of 2,6-Dimethylaniline, its isomeric impurities, and other related impurities by isocratic and reverse-phase ultra-performance liquid chromatographic (UPLC) method
- analyze a binary mixture of lidocaine hydrochloride and cetylpyridinium chloride in presence of lidocaine impurity A by spectrophotometric methods
- determine lidocaine hydrochloride-related substance by analytical methods in pharmaceutical dosage forms
Analysis Note
Footnote
Recommended products
Related product
signalword
Warning
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 2 - Carc. 2 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
wgk_germany
WGK 3
flash_point_f
195.8 °F - closed cup
flash_point_c
91 °C - closed cup
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico