391522
α-Methylhydrocinnamic acid
98%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
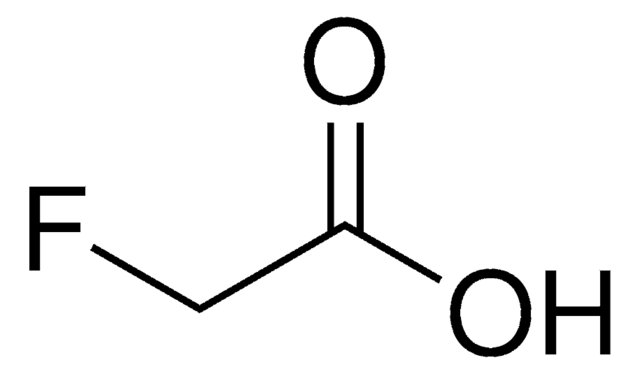
C6H5CH2CH(CH3)CO2H
Número de CAS:
Peso molecular:
164.20
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
assay
98%
form
solid
bp
167-168 °C/23 mmHg (lit.)
mp
39-41 °C (lit.)
density
1.065 g/mL at 25 °C (lit.)
functional group
carboxylic acid
phenyl
SMILES string
CC(Cc1ccccc1)C(O)=O
InChI
1S/C10H12O2/c1-8(10(11)12)7-9-5-3-2-4-6-9/h2-6,8H,7H2,1H3,(H,11,12)
InChI key
MCIIDRLDHRQKPH-UHFFFAOYSA-N
Categorías relacionadas
General description
α-Methylhydrocinnamic acid (2-methyl-3-phenylpropionic acid, 2-benzylpropionic acid) is a cinnamic acid derivative. Its synthesis by the asymmetric hydrogenation of α-methylcinnamic acid has been reported. α-Methylhydrocinnamic acid, a short chain fatty acid derivative (SCFAD), has been reported to correct the cystic fibrosis transmembrane conductance regulator (ΔF508-CFTR) defect. Conformational studies of 2-methyl-3-phenylpropionic acid has been investigated by NMR spectroscopy. The enantiomers of 2-benzylpropionic acid has been reported to be synthesized using a lipase-catalyzed resolution. (S) (+)-2-methyl-3-phenylpropionic acid participates in the synthesis of optically active (R)-5-methyl-6-phenylhexanoyl azide. L-2-Methyl-3.phenylpropionic acid has been reported to be an inhibitor of carboxypeptidase A. Polymer-supported “Evans” oxazolidinone mediated solid phase asymmetric has been employed in the synthesis of (S)-2-methyl-3-phenylpropionic acid.
Application
α-Methylhydrocinnamic acid is suitable for use in the comparative study to investigate the γ-globin inducibility of short-chain fatty acid derivatives (SCFADs) in mice. It may be used as a histone deacetylase (HDAC) inhibitor in the comparative study to investigate the EGFP-induction potency of a number of HDAC inhibitors. It may be used in the study to investigate the selectivity of the sensor based on imprinted poly(o-phenylenediamine) (iPoPD).
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
235.4 °F - closed cup
flash_point_c
113 °C - closed cup
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Stereochemical Studies. V. Intramolecular CH Bond Insertion Reaction of Acyl Nitrene generated from optically Active Acyl Azide.
Shiro T, et al.
Chemical & Pharmaceutical Bulletin, 18(6), 1124-1136 (1970)
Conformational studies of 2-methyl-3-phenylpropionic acid, 2-phenylbutyric acid and their methyl esters by NMR spectroscopy.
Spassov SL and Stefanova R.
Journal of Molecular Structure, 53, 219-224 (1979)
M T Tsamis et al.
Journal of chromatography, 277, 61-69 (1983-10-14)
Two chromatographic methods which allow the measurement of 2-phenylbutyric acid in serum are described: a gas chromatographic, after silylation, and a reversed-phase high-performance liquid chromatographic. The liquid chromatography with a fluorescent detection, after derivatization by 4-bromomethyl-7-methoxycoumarin, is ten times more
Yishan Liu et al.
Journal of hazardous materials, 192(3), 1633-1640 (2011-07-29)
Microbial degradation of the chiral 2-phenylbutyric acid (2-PBA), a metabolite of surfactant linear alkylbenzene sulfonates (LAS), was investigated using both racemic and enantiomer-pure compounds together with quantitative stereoselective analyses. A pure culture of bacteria, identified as Xanthobacter flavus strain PA1
Jörgen Samuelsson et al.
Journal of chromatography. A, 1163(1-2), 177-189 (2007-07-07)
A systematic study was made to explain the large improvements in separation performance and capacity of basic compounds at alkaline conditions. The adsorption of three probe components was investigated on four alkaline-stable silica-based C18 columns at three different pH-levels: 3
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico