196789
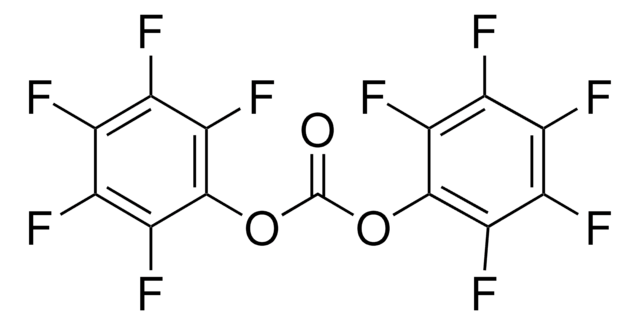
2,3,5,6-Tetrafluorophenol
97%
Sinónimos:
1,2,4,5-Tetrafluoro-3-hydroxybenzene
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
HC6F4OH
Número de CAS:
Peso molecular:
166.07
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de la sustancia en PubChem:
NACRES:
NA.22
Productos recomendados
Nivel de calidad
Ensayo
97%
Formulario
solid
bp
140 °C (lit.)
mp
37-39 °C (lit.)
grupo funcional
fluoro
cadena SMILES
Oc1c(F)c(F)cc(F)c1F
InChI
1S/C6H2F4O/c7-2-1-3(8)5(10)6(11)4(2)9/h1,11H
Clave InChI
PBYIIRLNRCVTMQ-UHFFFAOYSA-N
Categorías relacionadas
Aplicación
2,3,5,6-Tetrafluorophenol was used in preparation of :
- radioiodinated phenylalanine derivative which is useful in peptide synthesis
- technetium-99m labeled antibodies
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órganos de actuación
Respiratory system
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
174.2 °F - closed cup
Punto de inflamabilidad (°C)
79 °C - closed cup
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
A R Fritzberg et al.
Proceedings of the National Academy of Sciences of the United States of America, 85(11), 4025-4029 (1988-06-01)
Technetium-99m labeling of antibodies has been suboptimal because of low affinity adventitious binding, nonspecific labeling, and loss of immunoreactivity. The diamide dithiolate ligand system (N2S2) forms highly stable, well-defined tetradentate complexes with Tc(V). Antibodies and their fragments have been labeled
K Detmer et al.
The Journal of biological chemistry, 260(10), 5998-6005 (1985-05-25)
The oxidative half-reaction of phenol hydroxylase has been studied by stopped-flow spectrophotometry. Three flavin-oxygen intermediates can be detected when the substrate is thiophenol, or m-NH2, m-OH, m-CH3, m-Cl, or p-OH phenol. Intermediate I, the flavin C(4a)-hydroperoxide, has an absorbance maximum
D S Wilbur et al.
Bioconjugate chemistry, 4(6), 574-580 (1993-11-01)
An investigation to prepare a phenylalanine derivative which could be radioiodinated and used directly in peptide synthesis was conducted. N-Boc-p-(tri-n-butylstannyl)-L-phenylalanine tetrafluorophenyl ester was targeted and synthesized from N-Boc-p-iodo-L-phenylalanine. The requisite aryl stannylation reaction was found to be best conducted using
G A Eiceman et al.
Journal of the American Society for Mass Spectrometry, 10(11), 1157-1165 (2001-09-07)
Atmospheric pressure chemical ionization (APCI)-mass spectrometry (MS) for fluorinated phenols (C6H5-xFxOH Where x = 0-5) in nitrogen with Cl- as the reagent ion yielded product ions of M Cl- through ion associations or (M-H)- through proton abstractions. Proton abstraction was
C den Besten et al.
Chemical research in toxicology, 6(5), 674-680 (1993-09-01)
In the present study the oxidative dehalogenation of a para-halogenated phenol was studied using pentafluorophenol and its non-para-halogenated analogue 2,3,5,6-tetrafluorophenol as model compounds. 19F NMR was used to characterize the metabolite patterns. In order to study the primary oxidation products
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico