203290
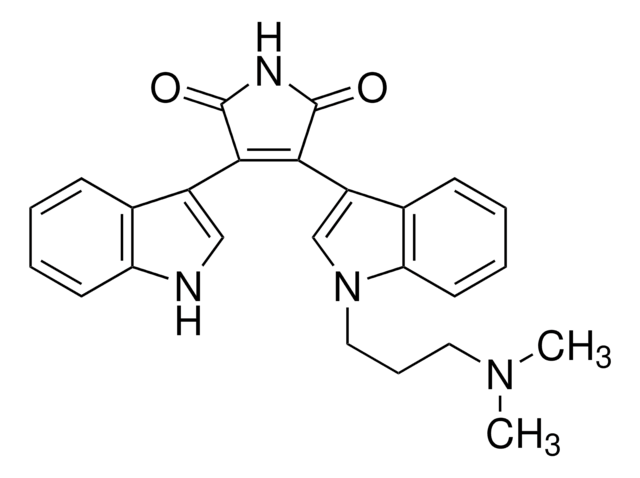
Bisindolylmaleimide I
A highly selective, cell-permeable, and reversible protein kinase C (PKC) inhibitor (IC₅₀ = 10 nM) that is structurally similar to staurosporine.
Sinónimos:
Bisindolylmaleimide I, 2-[1-(3-Dimethylaminopropyl)-1H-indol-3-yl]-3-(1H-indol-3-yl)-maleimide, Gö 6850, GF 109203X
About This Item
Productos recomendados
Nivel de calidad
Ensayo
≥95% (HPLC)
Formulario
solid
fabricante / nombre comercial
Calbiochem®
condiciones de almacenamiento
OK to freeze
protect from light
color
deep orange
solubilidad
DMSO: 10 mg/mL
Condiciones de envío
ambient
temp. de almacenamiento
2-8°C
cadena SMILES
N1C(=O)C(=C(C1=O)c4c5c([nH]c4)cccc5)c2c3c([n](c2)CCCN(C)C)cccc3
InChI
1S/C25H24N4O2/c1-28(2)12-7-13-29-15-19(17-9-4-6-11-21(17)29)23-22(24(30)27-25(23)31)18-14-26-20-10-5-3-8-16(18)20/h3-6,8-11,14-15,26H,7,12-13H2,1-2H3,(H,27,30,31)
Clave InChI
QMGUOJYZJKLOLH-UHFFFAOYSA-N
Descripción general
Acciones bioquímicas o fisiológicas
PKC
Advertencia
Nota de preparación
Reconstitución
Otras notas
Ku, W.-C., et al. 1997. Biochem. Biophys. Res. Commun. 241, 730.
Gekeler, V., et al. 1996. Br. J. Cancer 74, 897.
Kiss, Z., et al. 1995. Biochim. Biophys. Acta 1265, 93.
Toullec, D., et al. 1991. J. Biol. Chem. 266, 15771.
Información legal
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Learn tips and tricks for how to properly use inhibitors including how to select the right inhibitor and how to plan experiments with inhibitors.
Active Filters
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico