79417
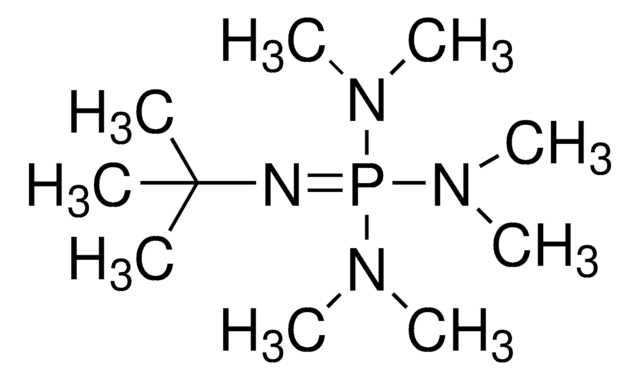
Phosphazene base P2-Et
≥98.0% (NT)
Sinónimos:
1-Ethyl-2,2,4,4,4-pentakis(dimethylamino)-2λ5,4λ5-catenadi(phosphazene), Tetramethyl(tris(dimethylamino)phosphoranylidene)phosphorictriamid-Et-imin
About This Item
Productos recomendados
Quality Level
assay
≥98.0% (NT)
form
liquid
refractive index
n20/D 1.492 (lit.)
n20/D 1.492
bp
96 °C/0.05 mmHg (lit.)
density
1.02 g/mL at 25 °C (lit.)
SMILES string
CCN=P(N=P(N(C)C)(N(C)C)N(C)C)(N(C)C)N(C)C
InChI
1S/C12H35N7P2/c1-12-13-20(15(2)3,16(4)5)14-21(17(6)7,18(8)9)19(10)11/h12H2,1-11H3
InChI key
CFUKEHPEQCSIOM-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
This strong non-ionic base has the ability to catalyze a wide variety of organic transformations such as:
- Palladium-catalyzed cross-coupling reactions when used in combination with tBuXPhos or tBuBrettPhos G3 precatalysts.
- Deprotonation of ortho-halobenzyl sulfones to generate α-sulfonyl benzylic carbanions.
- Conversion of vinyl sufone to allyl sulfone.
- α-Alkylation of 2-phenyl-2-oxazoline-4-carbonylcamphorsultam in the presence of tetrabutylammonium bromide.
signalword
Danger
hcodes
Hazard Classifications
Skin Corr. 1B
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Phosphazene base reagents are available as monomeric (P1 and BEMP), dimeric (P2), and tetrameric (P4) bases with different side chains to control their sterical hindrance.
Contenido relacionado
The Nicewicz lab is focused on the discovery of new and powerful reaction methodologies that proceed via the intermediacy of highly reactive cation radical species. Included in these transformations are anti-Markovnikov selective additions of amines, alcohols, carboxylic acids, amides and mineral acids to alkenes.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico





![1,8-Diazabiciclo[5.4.0]undec-7-eno 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)

![Tetrakis[tris(dimethylamino)phosphoranylidenamino]phosphonium chloride 95%](/deepweb/assets/sigmaaldrich/product/structures/160/963/9dd6d457-17b2-44dc-8ea2-d3c0475b3664/640/9dd6d457-17b2-44dc-8ea2-d3c0475b3664.png)