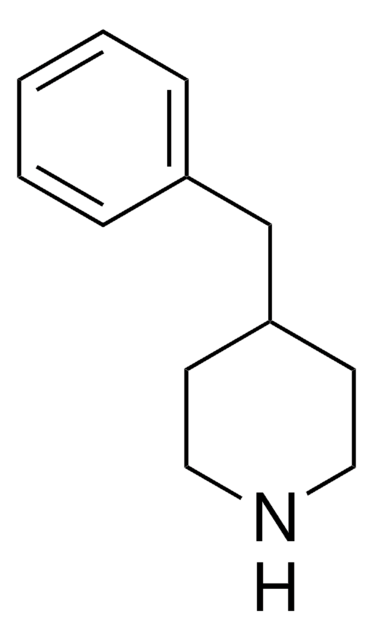
195812
4-Amino-1-benzylpiperidine
98%
Sinónimos:
1-(Phenylmethyl)-4-piperidinamine
About This Item
Productos recomendados
Nivel de calidad
Ensayo
98%
Formulario
liquid
índice de refracción
n20/D 1.543 (lit.)
densidad
0.933 g/mL at 25 °C (lit.)
grupo funcional
phenyl
cadena SMILES
NC1CCN(CC1)Cc2ccccc2
InChI
1S/C12H18N2/c13-12-6-8-14(9-7-12)10-11-4-2-1-3-5-11/h1-5,12H,6-10,13H2
Clave InChI
YUBDLZGUSSWQSS-UHFFFAOYSA-N
Información sobre el gen
rat ... Grin2a(24409)
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Aplicación
- butyl 4-amino-1-piperidineacetate, key intermediate required for the synthesis of butyl 4-[(4-amino-5-chloro-2-methoxybenzoyl)amino]-1-piperidineacetate
- 5-alkylimino-1,2,4-thiadiazolidine-3-ones, such as 4-ethyl-5-[imino-[1-(phenylmethyl)-4-piperidinyl]]-2-methyl-1,2,4-thiadiazolidin-3-one and 4-benzyl-5-[imino-[1-(phenylmethyl)-4-piperidinyl]]-2-isopropyl-1,2,4-thiadiazolidin-3-one
- glycyrrhetinic acid derivatives
Highly selective inhibitors of p38a mitogen-activated protein kinase
Antiplasmodial compounds
Dual activity cholinesterase and Aβ-aggregation inhibitors
Muscarinic acetylcholine receptor antagonist and beta 2 adrenoceptor agonists
Photostable near-infrared cyanine dyes
99mTc-labeled piperidine analogues for targeting sigma receptors
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Skin Irrit. 2
Código de clase de almacenamiento
10 - Combustible liquids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
230.0 °F - closed cup
Punto de inflamabilidad (°C)
110 °C - closed cup
Equipo de protección personal
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico