SML1950
UVI3003
≥98% (HPLC)
Sinonimo/i:
3-[4-Hydroxy-3-[5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-3-(pentyloxy)-2-naphthalenyl]phenyl]-2-propenoic acid
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
≥98% (HPLC)
Forma fisica
powder
Colore
white to beige
Solubilità
DMSO: 20 mg/mL, clear
Temperatura di conservazione
−20°C
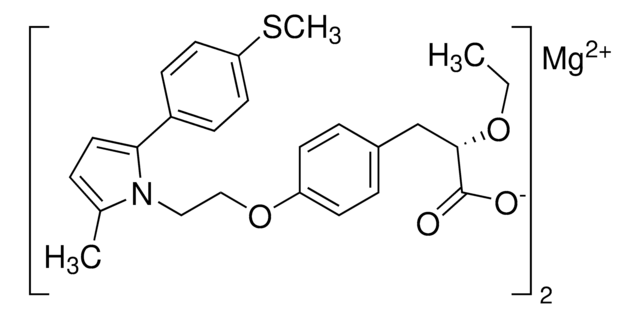
Stringa SMILE
CC(CCC1(C)C)(C)C2=C1C=C(C3=C(O)C=CC(C=CC(O)=O)=C3)C(OCCCCC)=C2
InChI
1S/C28H36O4/c1-6-7-8-15-32-25-18-23-22(27(2,3)13-14-28(23,4)5)17-21(25)20-16-19(9-11-24(20)29)10-12-26(30)31/h9-12,16-18,29H,6-8,13-15H2,1-5H3,(H,30,31)/b12-10+
APJSHECCIRQQDV-ZRDIBKRKSA-N
Azioni biochim/fisiol
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.