03449
N-(3-dimetilaminopropil)-N′-etilcarbodiimide
≥99.0% (AT)
Sinonimo/i:
N-etil-N′-(3-dimetilaminopropil)carbodiimide, EDAC, EDC, WSC
About This Item
Prodotti consigliati
Saggio
≥99.0% (AT)
Forma fisica
powder
tecniche
bioconjugation: suitable
Punto di fusione
110-115 °C (lit.)
112-116 °C
Solubilità
H2O: soluble 0.2 g/L
Temperatura di conservazione
−20°C
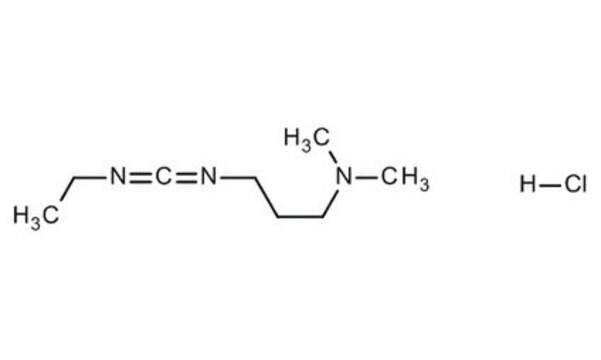
Stringa SMILE
Cl.CCN=C=NCCCN(C)C
InChI
1S/C8H17N3.ClH/c1-4-9-8-10-6-5-7-11(2)3;/h4-7H2,1-3H3;1H
FPQQSJJWHUJYPU-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Furthermore, EDAC HCl serves as a biomolecule bridge, acting as a crosslinker that connects amine-reactive NHS-esters of biomolecules to carboxyl groups. This technique proves invaluable in protein conjugation, enabling the creation of hybrid molecules with novel properties and functions. The underlying mechanism involves EDAC HCl′s reaction with a carboxyl group, forming an unstable intermediate that actively seeks an amine partner. The delicate balance of this reaction underscores the importance of optimizing conditions for efficient conjugation. The assistance of N-hydroxysuccinimide (NHS) further enhances EDAC HCl′s capabilities by stabilizing the intermediate and enabling two-step conjugation procedures. This additional feature provides greater flexibility and control, particularly when dealing with complex biomolecules.
Applicazioni
Azioni biochim/fisiol
Caratteristiche e vantaggi
Altre note
Prodotto comparabile
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT RE 2 Oral
Organi bersaglio
Stomach,large intestine,lymph node
Codice della classe di stoccaggio
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Professor Aran discusses engineering graphene-based materials through careful functionalization, enabling diverse applications.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.








![1-[3-(Dimethylamino)propyl]-3-ethylcarbodiimide methiodide](/deepweb/assets/sigmaaldrich/product/structures/414/134/4eb9c126-d7f9-4e12-9e3a-95cb077824fd/640/4eb9c126-d7f9-4e12-9e3a-95cb077824fd.png)



