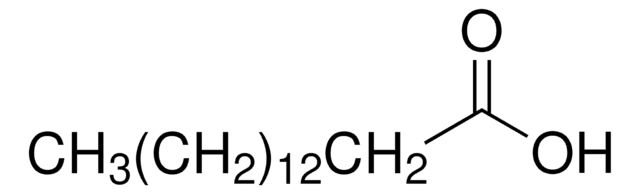P7754
Acetilacetone
ReagentPlus®, ≥99%
Sinonimo/i:
2,4-pentanedione
About This Item
Prodotti consigliati
Densità del vapore
3.5 (vs air)
Livello qualitativo
Tensione di vapore
6 mmHg ( 20 °C)
Nome Commerciale
ReagentPlus®
Saggio
≥99%
Forma fisica
liquid
Temp. autoaccensione
662 °F
Limite di esplosione
11.4 %
Indice di rifrazione
n20/D 1.452 (lit.)
pH
6 (20 °C, 200 g/L)
P. eboll.
140.4 °C (lit.)
Punto di fusione
−23 °C (lit.)
Densità
0.975 g/mL at 25 °C (lit.)
Stringa SMILE
CC(=O)CC(C)=O
InChI
1S/C5H8O2/c1-4(6)3-5(2)7/h3H2,1-2H3
YRKCREAYFQTBPV-UHFFFAOYSA-N
Informazioni sul gene
human ... ACHE(43) , BCHE(590) , CES1(1066)
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
- A multifunctional ligand in the synthesis and feasible functionalization of gold nanoparticles (AuNPs).
- A reactant to synthesize 9,10-dihydroacridines by reacting with methyl acetoacetate and Morita-Baylis-Hillman acetates.
- A reagent in the synthesis of ZrO2(zirconium dioxide) via hydrolysis of Zr(OC3H7n)4. Acetylacetone controls the hydrolysis and condensation rates of alkoxides and thus, the nucleation and growth rates of oxides.
Confezionamento
Note legali
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 4 Oral - Flam. Liq. 3
Codice della classe di stoccaggio
3 - Flammable liquids
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
95.0 °F - closed cup
Punto d’infiammabilità (°C)
35 °C - closed cup
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.