665290
(S,S,S)-(+)-(3,5-Dioxa-4-phosphacyclohepta[2,1-a:3,4-a′]dinaphthalen-4-yl)bis(1-phenylethyl)amine
97%
Sinonimo/i:
(+)-N,N-Bis[(1S)-1-phenylethyl]- dinaphtho[2,1-d:1′,2′-f][1,3,2]dioxaphosphepin- 4-amine, (11bR)
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
97%
Stato
solid
Punto di fusione
88-89 °C
Gruppo funzionale
amine
phenyl
Stringa SMILE
C[C@H](N([C@@H](C)c1ccccc1)P2Oc3ccc4ccccc4c3-c5c(O2)ccc6ccccc56)c7ccccc7
InChI
1S/C36H30NO2P/c1-25(27-13-5-3-6-14-27)37(26(2)28-15-7-4-8-16-28)40-38-33-23-21-29-17-9-11-19-31(29)35(33)36-32-20-12-10-18-30(32)22-24-34(36)39-40/h3-26H,1-2H3/t25-,26-/m0/s1
LKZPDRCMCSBQFN-UIOOFZCWSA-N
Categorie correlate
Applicazioni
- Iridium-catalyzed allylic etherification of acyclic, achiral allylic carbonates with potassium silanolates to form chiral allylic alcohols.
- Palladium-catalyzed asymmetric allylic cyclisation of N-tosyl and N-benzyl carbonates to form the corresponding pyrrolidine and piperidine derivatives, respectively.
- Intramolecular iridium-catalyzed allylic cyclizationof (E)-allylic methyl carbonates to form 2,5-trans/cis pyrrolidine derivatives.
Caratteristiche e vantaggi
- Superior enantiocontrol in numerous transformations
- High activities at low catalyst loadings
- Hydrogenations under low-pressure conditions
Note legali
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
DSM collaboration offers MonoPhos™ ligands for research, based on the BINOL platform by Feringa and co-workers.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.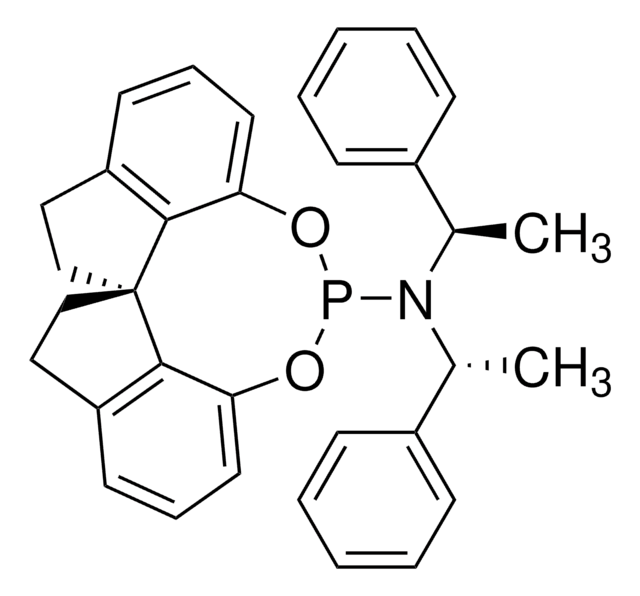
![(S,R,R)-(+)-(3,5-Dioxa-4-phosphacyclohepta[2,1-a:3,4-a′]dinaphthalen-4-yl)bis(1-phenylethyl)amine 95% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/366/790/7555ef31-5d0b-45c9-ad40-5dfd0fe85125/640/7555ef31-5d0b-45c9-ad40-5dfd0fe85125.png)
![(S)-(+)-N-(3,5-Dioxa-4-phosphacyclohepta[2,1-a;3,4-a′]dinaphthalen-4-yl)-dibenzo[b,f]azepine ≥95% (elemental analysis)](/deepweb/assets/sigmaaldrich/product/structures/575/489/d54360f9-5a59-43f2-bc44-42f5fa92b588/640/d54360f9-5a59-43f2-bc44-42f5fa92b588.png)
![(S)-(+)-(3,5-Dioxa-4-phosphacyclohepta[2,1-a;3,4- a′]dinaphthalen-4-yl)dimethylamine 97%](/deepweb/assets/sigmaaldrich/product/structures/400/008/628143de-3954-440a-ba9c-4c0ff8e44663/640/628143de-3954-440a-ba9c-4c0ff8e44663.png)
![1,8-diazabiciclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)
![4,7,13,16,21,24-Hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane 98%](/deepweb/assets/sigmaaldrich/product/structures/189/812/8a6555e5-8de6-4236-865f-19339cee3634/640/8a6555e5-8de6-4236-865f-19339cee3634.png)

![(S,R)-(+)-(3,5-Dioxa-4-phospha-cyclohepta[2,1-a;4-a′]dinaphthalen-4-yl)-(1-phenylethyl)amine 96%](/deepweb/assets/sigmaaldrich/product/structures/340/157/5071e653-a834-4559-9aa7-4eb2d3774e42/640/5071e653-a834-4559-9aa7-4eb2d3774e42.png)


![(−)-Bis[(S)-1-phenylethyl]amine 99%](/deepweb/assets/sigmaaldrich/product/structures/336/455/d6f04f0e-9bcc-4d67-a94d-d153e39209e1/640/d6f04f0e-9bcc-4d67-a94d-d153e39209e1.png)