493937
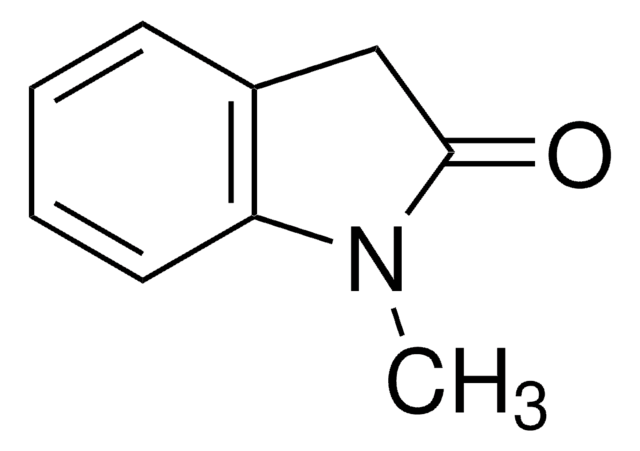
3-Methyl-2-oxindole
96%
Sinonimo/i:
3-Methyloxindole
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C9H9NO
Numero CAS:
Peso molecolare:
147.17
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
96%
Forma fisica
solid
Punto di fusione
117-121 °C (lit.)
Stringa SMILE
CC1C(=O)Nc2ccccc12
InChI
1S/C9H9NO/c1-6-7-4-2-3-5-8(7)10-9(6)11/h2-6H,1H3,(H,10,11)
BBZCPUCZKLTAJQ-UHFFFAOYSA-N
Categorie correlate
Descrizione generale
3-Methyl-2-oxindole (MOI) is a 3-substituted-2-oxindole. It is reported to be formed during the oxidation of indole-3-acetic acid in the presence of FeII under aerobic conditions. MOI undergoes asymmetric anti-Mannich-type reaction with N-tosyl aryl aldimines in the presence of alkaloid cinchona derivatives to form anti-3,3-disubsituted 2-oxindole derivatives. It also undergoes asymmetric hydroxyamination with nitrosoarenes to form N-nitroso aldol products.
Applicazioni
3-Methyl-2-oxindole may be used in the preparation of 3-hydroxy-3-methyl-2-oxindole.
- Reactant for enantioselective α-amination reactions
- Reactant for aldol reaction with glyoxal derivatives
- Reactant for amine thiourea catalyzed conjugate addition to α,β-unsaturated aldehydes
- Reactant for O-acetylation reactions
- Reactant for preparation of a disubstituted oxoindole by using rhodium-catalyzed cyclopropanation/ring-opening reactions
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Ying Jin et al.
Chirality, 26(12), 801-805 (2014-07-22)
A series of cinchona alkaloid derivatives were used to catalyze the asymmetric anti-Mannich-type reaction of 3-methyl-2-oxindole with N-tosyl aryl aldimines. The resulting anti-3,3-disubstituted 2-oxindole products were obtained in good yields (up to 92%) with high diastereo- and enantioselectivities (anti/syn up
Metabolism and pneumotoxicity of 3-methyloxindole, indole-3-carbinol, and 3-methylindole in goats.
M J Potchoiba et al.
American journal of veterinary research, 43(8), 1418-1423 (1982-08-01)
Facile and Efficient Enantioselective Hydroxyamination Reaction: Synthesis of 3-Hydroxyamino-2-Oxindoles Using Nitrosoarenes.
Shen K, et al.
Angewandte Chemie (Weinheim an der Bergstrasse, Germany), 123(20), 4780-4784 (2011)
J Thornton-Manning et al.
The Journal of pharmacology and experimental therapeutics, 276(1), 21-29 (1996-01-01)
The toxicity of 3-methylindole (3 MI), a selective pneumotoxin, is dependent upon cytochrome P450-mediated bioactivation 3. Using vaccinia-expressed P450 enzymes, the metabolites of radiolabeled 3 MI produced by 14 individual P450s were identified and quantified by high performance liquid chromatography.
Jaroslav Matal et al.
Neuro endocrinology letters, 30 Suppl 1, 36-40 (2009-12-23)
To study the contribution of individual purified porcine CYP1A2, 2E1 and 2A19 enzymes to the biotransformation of skatole. Individual porcine and human enzymes (CYP1A2, 2E1 or 2A6/19) were used to study their potential involvement in skatole metabolism. Furthermore, the inhibition
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.