424048
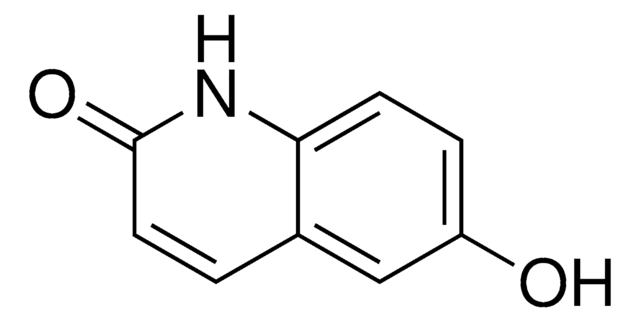
2,6-Quinolinediol
Sinonimo/i:
2,6-Dihydroxyquinoline, 6-Hydroxycarbostyril
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C9H7NO2
Numero CAS:
Peso molecolare:
161.16
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Punto di fusione
>300 °C (lit.)
Stringa SMILE
Oc1ccc2nc(O)ccc2c1
InChI
1S/C9H7NO2/c11-7-2-3-8-6(5-7)1-4-9(12)10-8/h1-5,11H,(H,10,12)
AQLYZDRHNHZHIS-UHFFFAOYSA-N
Descrizione generale
2,6-Quinolinediol is a substituted quinoline compound. It has been prepared starting from 6-methoxyquinoline. It has been reported to be one of the UV-absorbing compounds (UAC) found in cow milk.
Applicazioni
2,6-Quinolinediol may be used as a test compound to investigate the carcinogenicity of the naturally occurring quinoline metabolite of tryptophan. The study was conducted by suspending tryptophan metabolites in cholesterol pellets, followed by the surgical implantation of the pellets into the mice bladder. It may be used as a reactant in the synthesis of 2-chloro-6-quinolinol by reacting with POCl3 (phosphorus oxychloride).
Reactant involved in synthesis of:
- (Benzyloxy)methylquinolinone derivatives for use as PDE3 inhibitors
- Substances related to cilostazol
- 2,6-Quinolinyl derivatives for use as VLA-4 / VCAM-1 antagonists
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Mouse bladder carcinogenicity of certain tryptophan metabolites and other aromatic nitrogen compounds suspended in cholesterol.
Bryan GT, et al.
Cancer Research, 24(4), 596-602 (1964)
2, 6-Quinolinyl derivatives as potent VLA-4 antagonists.
Lassoie MA, et al.
Bioorganic & Medicinal Chemistry Letters, 17(1), 142-146 (2007)
In vivo elution of tryptophan metabolites and other aromatic nitrogen compounds from cholesterol pellets implanted into mouse bladders.
Bryan GT, et al.
Cancer Research, 24(4), 586-595 (1964)
Pascal Rouge et al.
Food chemistry, 141(3), 1888-1894 (2013-07-23)
The aim of this work was to characterise new UV-absorbing compounds (UAC) in cow milk in order to gain an overview of the molecular diversity of the minor bioactive constituents, that could be used to trace animal feed or that
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.