SML3203
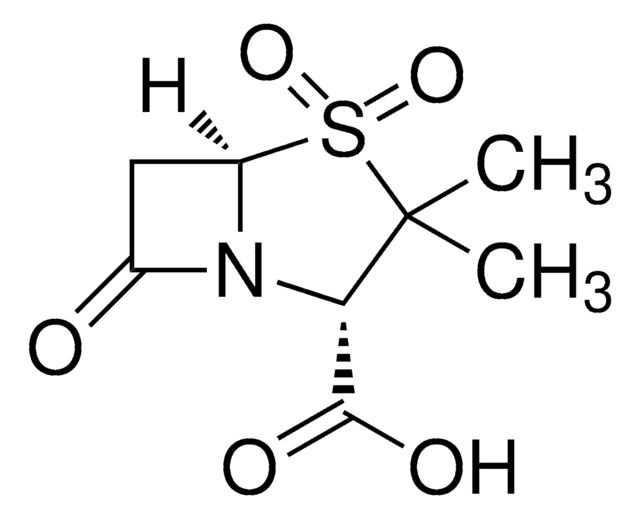
Tebipenem
≥98% (HPLC)
Synonym(e):
(1R,5S,6S)-2-[1-(1,3-Thiazolin-2-yl)azetidin-3-yl]thio-6-[(R)-1-hydroxyeth yl]-1-methyl-carbapen-2-em-3-carboxylic acid, (4R,5S,6S)-3-[[1-(4,5-Dihydro-2-thiazolyl)-3-azetidinyl]thio]-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid, L-084 active drug, LJC 11,036, LJC 11036, ME1211 active drug, SPR 859, SPR 994 active drug, SPR-859, SPR-994 active drug, SPR859, SPR994 active drug, TBM, TBM-PI active drug, Tebi-pivoxil active drug, [4R-[4α,5β,6β(R*)]]-3-[[1-(4,5-Dihydro-2-thiazolyl)-3-azetidinyl]thio]-6-(1-hydroxyethyl)-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
≥98% (HPLC)
Form
powder
Farbe
white to beige
Löslichkeit
DMSO: 2 mg/mL, clear
Lagertemp.
−20°C
SMILES String
S1CCN=C1N2CC(C2)SC3=C(N4[C@H]([C@H]3C)[C@H](C4=O)[C@H](O)C)C(=O)O
InChI
1S/C16H21N3O4S2/c1-7-11-10(8(2)20)14(21)19(11)12(15(22)23)13(7)25-9-5-18(6-9)16-17-3-4-24-16/h7-11,20H,3-6H2,1-2H3,(H,22,23)/t7-,8-,10-,11-/m1/s1
InChIKey
GXXLUDOKHXEFBQ-YJFSRANCSA-N
Biochem./physiol. Wirkung
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Analysenzertifikate (COA)
Die passende Version wird nicht angezeigt?
Wenn Sie eine bestimmte Version benötigen, können Sie anhand der Lot- oder Chargennummer nach einem spezifischen Zertifikat suchen.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Active Filters
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.