H7002
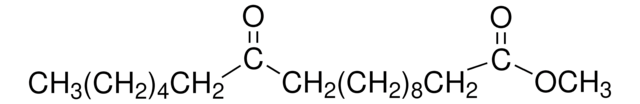
Methyl-12-hydroxystearat
≥99% (GC)
Synonym(e):
12-Hydroxystearinsäure-methylester, Methyl-12-hydroxyoctadecanoat
About This Item
Empfohlene Produkte
Assay
≥99% (GC)
Form
powder
Funktionelle Gruppe
ester
Lipid-Typ
saturated FAs
Versandbedingung
ambient
Lagertemp.
−20°C
SMILES String
CCCCCCC(O)CCCCCCCCCCC(=O)OC
InChI
1S/C19H38O3/c1-3-4-5-12-15-18(20)16-13-10-8-6-7-9-11-14-17-19(21)22-2/h18,20H,3-17H2,1-2H3
InChIKey
RVWOWEQKPMPWMQ-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Anwendung
- Chemical Changes of Hydroperoxy-, Epoxy-, Keto- and Hydroxy-Model Lipids under Simulated Gastric Conditions.: This study explores the stability and chemical transformations of hydroxy fatty acids, including Methyl 12-hydroxystearate, under digestive conditions, providing insight into dietary fat metabolism and its implications for nutritional sciences (Marquez-Ruiz et al., 2021).
- Stimulation of nitrogen removal in the rhizosphere of aquatic duckweed by root exudate components.: This research highlights the potential environmental applications of Methyl 12-hydroxystearate, as a standard, in enhancing nitrogen cycling, important for studies on wastewater treatment and ecosystem management (Lu et al., 2014).
- Synthesis and evaluation of antioxidant and antifungal activities of novel ricinoleate-based lipoconjugates of phenolic acids.: This study investigates the synthesis of derivatives of Methyl 12-hydroxystearate for potential use in food preservation and pharmaceutical applications, emphasizing its antioxidant and antifungal properties (Reddy et al., 2012).
Biochem./physiol. Wirkung
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.