806536
APN-Maleimide
Synonym(e):
3-(4-(2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl)phenyl)propiolonitrile, MAPN
About This Item
Empfohlene Produkte
Form
powder
Qualitätsniveau
Lagertemp.
2-8°C
SMILES String
N#CC#CC1=CC=C(N2C(C=CC2=O)=O)C=C1
InChI
1S/C13H6N2O2/c14-9-1-2-10-3-5-11(6-4-10)15-12(16)7-8-13(15)17/h3-8H
InChIKey
CHKKXKRQICWZFF-UHFFFAOYSA-N
Anwendung
Angaben zur Herstellung
- Dissolve the protein in the appropriate buffer* with pH 6.5-9.0 (e.g. PBS) at 1-10 mg/mL concentration.
- Apply the appropriate amount of the stock solution of the reagent (1-5 molar eq. per free cysteine residue).
- Incubate at room temperature for 10 minutes.
- If necessary, purify the protein-APN conjugate using size exclusion chromatography or ultrafiltration.
- The conjugate can be readily coupled with thiol-containing substrates by incubating the components in aqueous buffer (pH 6.5-9.0) at ambient temperature for 2 hours.
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.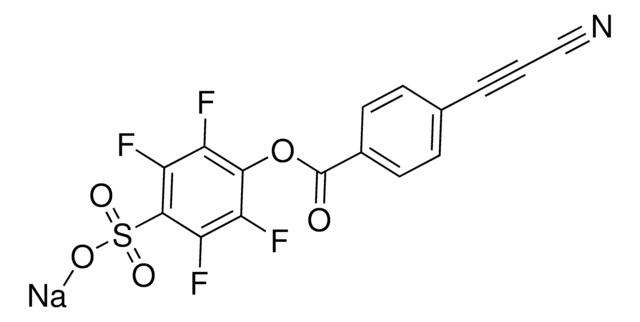










![(1R,8S,9s)-Bicyclo[6.1.0]non-4-in-9-ylmethyl]-N-succinimidylcarbonat for Copper-free Click Chemistry](/deepweb/assets/sigmaaldrich/product/structures/969/022/d6776082-2f7a-47c7-bcd4-3830dac0fb7d/640/d6776082-2f7a-47c7-bcd4-3830dac0fb7d.png)