763314
sSPhos Pd G2
95%
Synonym(e):
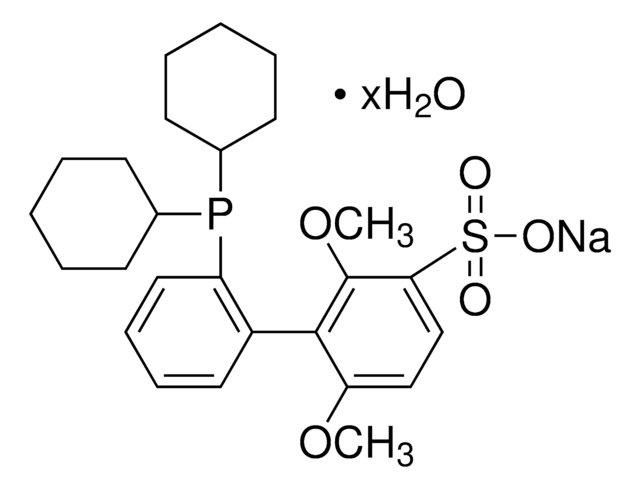
Chloro(sodium-2-dicyclohexylphosphino-2′,6′-dimethoxy-1,1′-biphenyl-3′-sulfonate)[2-(2′-amino-1,1′-biphenyl)]palladium(II)
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
95%
Form
solid
Leistungsmerkmale
generation 2
Eignung der Reaktion
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
reaction type: Cross Couplings
mp (Schmelzpunkt)
193-194 °C (decomposition)
Funktionelle Gruppe
phosphine
SMILES String
[Na+].Nc1ccccc1-c2ccccc2[Pd]Cl.COc3ccc(c(OC)c3-c4ccccc4P(C5CCCCC5)C6CCCCC6)S([O-])(=O)=O
InChI
1S/C26H35O5PS.C12H10N.ClH.Na.Pd/c1-30-22-17-18-24(33(27,28)29)26(31-2)25(22)21-15-9-10-16-23(21)32(19-11-5-3-6-12-19)20-13-7-4-8-14-20;13-12-9-5-4-8-11(12)10-6-2-1-3-7-10;;;/h9-10,15-20H,3-8,11-14H2,1-2H3,(H,27,28,29);1-6,8-9H,13H2;1H;;/q;;;2*+1/p-2
InChIKey
MWQYWXJKMDRSJN-UHFFFAOYSA-L
Anwendung
- DNA-compatible Suzuki-Miyaura reaction between DNA-linked aryl halides and various boronic acids/esters including heteroaryl boronates.
- DNA-compatible hydroxycarbonylation reactions.
- The cross-coupling reaction between boronic ester and brominated cyclic nucleotide to synthesize cyclic adenosine and guanosine monophosphate in the presence of potassium phosphate and hydrochloric acid.
Ähnliches Produkt
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
Buchwald precatalysts and ligands are bulky electron-rich dialkylbiaryl phospine-based catalysts and structurally related ligands that improve reactivity in Pd-catalyzed cross - coupling reactions
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.
![Chloro-(2-dicyclohexylphosphino-2′,6′-dimethoxy-1,1′-biphenyl)[2-(2-aminoethylphenyl)]-palladium(II) - Methyl-t-butylether Addukt](/deepweb/assets/sigmaaldrich/product/structures/421/182/4ca66fb3-8d93-499c-b88b-b8fe48ca97b8/640/4ca66fb3-8d93-499c-b88b-b8fe48ca97b8.png)




![[Pd(OAc)2]3 reagent grade, 98%](/deepweb/assets/sigmaaldrich/product/structures/508/249/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f/640/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f.png)


![Cucurbit[7]uril contains acid of crystalization](/deepweb/assets/sigmaaldrich/product/structures/334/498/b3862b3d-15de-460c-ac7c-dd8a137c101d/640/b3862b3d-15de-460c-ac7c-dd8a137c101d.png)





