Alle Fotos(1)
Wichtige Dokumente
511188
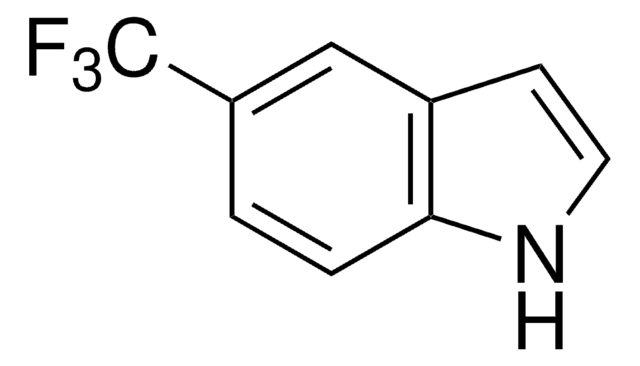
Methylindol-5-carboxylat
99%
Synonym(e):
Indole-5-carboxylic acid, methyl ester
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C10H9NO2
CAS-Nummer:
Molekulargewicht:
175.18
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
99%
mp (Schmelzpunkt)
126-128 °C (lit.)
Funktionelle Gruppe
ester
SMILES String
COC(=O)c1ccc2[nH]ccc2c1
InChI
1S/C10H9NO2/c1-13-10(12)8-2-3-9-7(6-8)4-5-11-9/h2-6,11H,1H3
InChIKey
DRYBMFJLYYEOBZ-UHFFFAOYSA-N
Verwandte Kategorien
Allgemeine Beschreibung
Methyl indole-5-carboxylate (Methyl 1H-indole-5-carboxylate), a substituted 1H-indole, can be prepared by the esterification of indole-5-carboxylic acid. Its efficacy as a substrate for indigoid generation has been assessed.
Anwendung
Methyl indole-5-carboxylate may be used as a reactant in the following processes:
- biosynthesis of inhibitors of protein kinases
- metal-free Friedel-Crafts alkylation
- preparation of diphenylsulfonium ylides from Martin′s sulfurane
- cross dehydrogenative coupling reactions
- synthesis of indirubin derivatives
- preparation of aminoindolylacetates
Methyl indole-5-carboxylate may be used in the preparation of:
- methyl indoline-5-carboxylate
- 1H-indole-5-carbohydrazide
- dimethyl 1H-indole-3,5-dicarboxylate
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
A direct ylide transfer to carbonyl derivatives and heteroaromatic compounds.
Xueliang Huang et al.
Angewandte Chemie (International ed. in English), 49(47), 8979-8983 (2010-10-13)
Wen-Jie Lu et al.
European journal of medicinal chemistry, 64, 498-511 (2013-05-21)
This report describes the synthesis, and in vitro and in vivo antimalarial evaluations of certain ester-modified neocryptolepine (5-methyl-5H-indolo[2,3-b]quinoline) derivatives. The modifications were carried out by introducing ester groups at the C2 and/or C9 position on the neocryptolepine core and the
Fei Yang et al.
Organic letters, 12(22), 5214-5217 (2010-10-23)
A novel cross dehydrogenative coupling (CDC) reaction of N,N-dimethylanilines with methyl ketones by cooperative copper and aminocatalysis has been developed, which leads to the formation of β-arylamino ketones in 42-73% yields. Moreover, the copper-catalyzed alkylation of free (NH) indoles with
Wang, T. C.; et al.
Chinese Chemical Letters = Zhongguo Hua Xue Kuai Bao, 21, 1407-1407 (2010)
Liu, Z.; et al.
Letters in Organic Chemistry, 7, 666-666 (2010)
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.