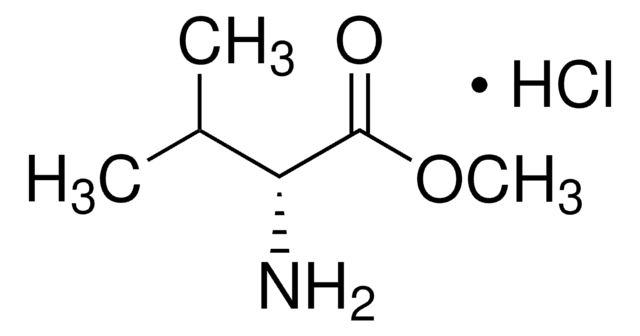
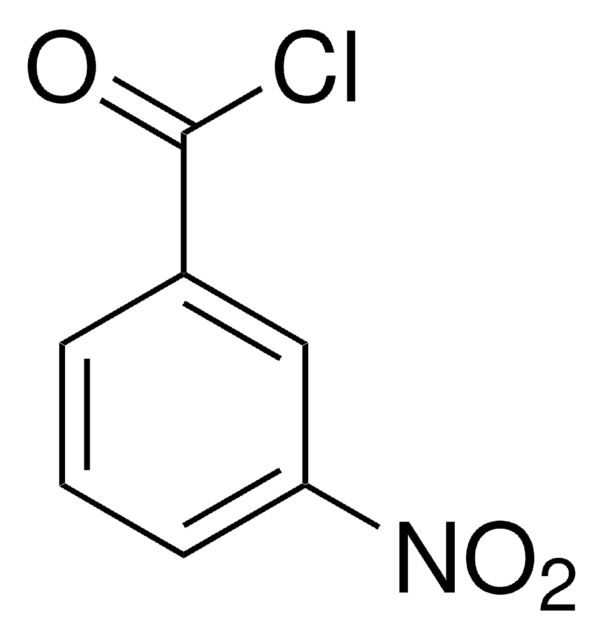
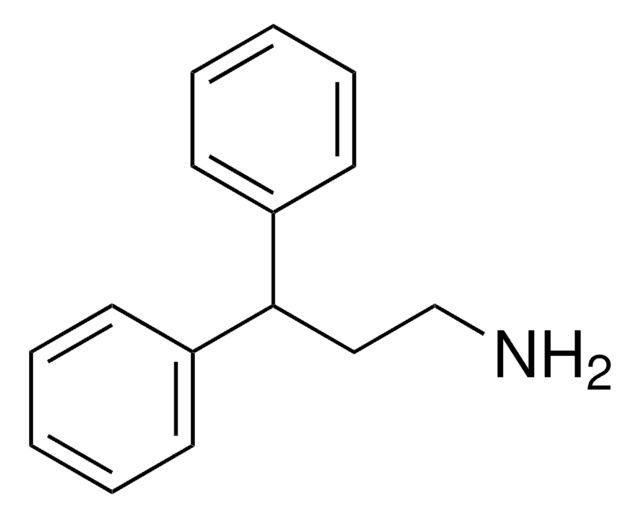
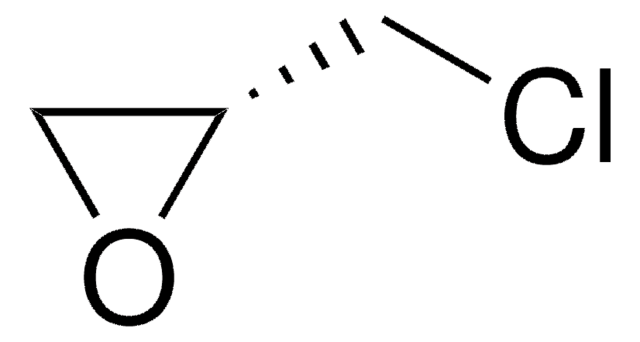
73032AST
Astec® CHIRALDEX™ G-TA Capillary GC Column
L × I.D. 20 m × 0.25 mm, df 0.12 μm
About This Item
Produtos recomendados
Materiais
fused silica
Nível de qualidade
descrição
GC capillary column
embalagem
pkg of 1 ea
Parâmetros
-10-180 °C temperature (isothermal or programmed)
Valor beta
500
df
0.12 μm
técnica(s)
gas chromatography (GC): suitable
C × D.I.
20 m × 0.25 mm
Grupo ativo da matriz
non-bonded; 2,6-di-O-pentyl-3-trifluoroacetyl derivative of γ-cyclodextrin phase
aplicação(ões)
agriculture
chemicals and industrial polymers
cleaning products
clinical
cosmetics
environmental
flavors and fragrances
food and beverages
forensics and toxicology
life science and biopharma
personal care
pharmaceutical (small molecule)
tipo de coluna
capillary chiral
técnica de separação
chiral
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
Resistência química/física
- -10 °C to 180 °C isothermal and programmed
Outras notas
Informações legais
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Artigos
Chromatographic enantiomeric separation of amino acids, like proline, is described for chiral GC analysis after derivatization.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica


![5-Boc-1,4,6,7-tetrahydro-pyrazolo[4,3-c]pyridine-3-carboxylic acid](/deepweb/assets/sigmaaldrich/product/structures/145/700/2ac352d1-30ff-42c1-b2f3-0fe11cecf36f/640/2ac352d1-30ff-42c1-b2f3-0fe11cecf36f.png)