SRP6316
Antithrombin III from human plasma
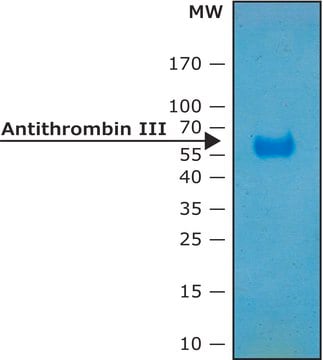
≥95% (SDS-PAGE)
Sinônimo(s):
SERPINC1
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Código UNSPSC:
12352202
NACRES:
NA.32
Produtos recomendados
fonte biológica
human
Ensaio
≥95% (SDS-PAGE)
Formulário
lyophilized
peso molecular
58 kDa
embalagem
pkg of 100 μg
pkg of 500 μg
nº de adesão UniProt
Condições de expedição
wet ice
temperatura de armazenamento
−20°C
Informações sobre genes
human ... SERPINC1(462)
Descrição geral
SerpinC1, also known as antithrombin III (AT III), is a member of the serpin superfamily of serine protease inhibitors. The gene is mapped to human chromosome 1. SerpinC1 is a glycoprotein with three β-sheets and nine α-helices. The protein has an active site region and a heparin binding site.
Ações bioquímicas/fisiológicas
SerpinC1, also known as antithrombin III (AT III), has been found to be a marker for disseminated intravascular coagulation (DIC) and to be of prognostic significance in septic patients. Antithrombin III synthesized in the liver is the principal plasma serpin of blood coagulation proteases and inhibits thrombin and other factors such as Xa by the formation of covalently linked complexes. Thus, it is one of the most important coagulation inhibitors and the fundamental enzyme for the therapeutical action of heparin. The inhibitory activity of AT III undergoes a dramatic increase in the presence of heparin and other glycosaminoglycans. Antithrombin III mediates the promotion of prostaglandin release, an inhibitor of leucocyte activation and downregulation of many proinflammatory cytokines. It exerts anti-inflammatory properties in addition to its anti-coagulative mechanisms. The deficiency or functional abnormality of AT III may result in an increased risk of thromboembolic disease, such as deep vein thrombosis and pulmonary embolism. In addition, it has been reported that AT III can alter or influence inflammatory processes via inhibition of NF (nuclear factor)-κB activation or actin polymerization. It is found in normal serum at 15mg/100mL. Found at higher levels in plasma than in serum because of complexing with thrombin during coagulation. Clinically, reduced levels are indicative of hypercoagulability. Reduction in the levels of antithrombin III enhances the severity of renal ischemia/reperfusion injury.
forma física
Lyophilized from 50 mM Tris-HCl, pH 8.0, with 150 mM NaCl.
Reconstituição
In water or aqueous buffer
Código de classe de armazenamento
13 - Non Combustible Solids
Classe de risco de água (WGK)
nwg
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Certificados de análise (COA)
Lot/Batch Number
Não está vendo a versão correta?
Se precisar de uma versão específica, você pode procurar um certificado específico pelo número do lote ou da remessa.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
The anti-inflammatory properties of antithrombin III: new therapeutic implications.
Okajima K and Uchiba M
Seminars in Thrombosis and Hemostasis, 24, 27-32 (1998)
Antithrombin III supplementation in severe sepsis: beneficial effects on organ dysfunction.
Inthorn D, et al.
Shock, 8, 328-334 (1997)
Laboratory tests for antithrombin deficiency.
Khor B and Van Cott EM
American Journal of Hematology, 85, 947-950 (2010)
Antithrombin III inhibits nuclear factor kappaB activation in human monocytes and vascular endothelial cells.
Oelschlager C, et al.
Blood, 99, 4015-4020 (2002)
Plasma antithrombin III and thrombin generation time: correlation with hemoglobin A1 and fasting serum glucose in young diabetic women.
Sowers JR, et al.
Diabetes Care, 3, 655-658 (1980)
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica