About This Item
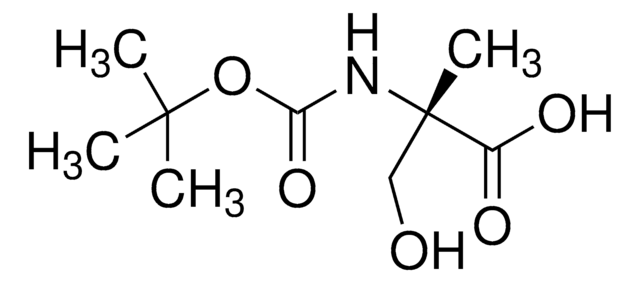
Fórmula empírica (Notação de Hill):
C4H9NO3
Número CAS:
Peso molecular:
119.12
Número CE:
Número MDL:
Código UNSPSC:
12352200
ID de substância PubChem:
NACRES:
NA.26
Produtos recomendados
Ensaio
≥98% (TLC)
Formulário
powder
cor
white
cadeia de caracteres SMILES
CC(N)(CO)C(O)=O
InChI
1S/C4H9NO3/c1-4(5,2-6)3(7)8/h6H,2,5H2,1H3,(H,7,8)
chave InChI
CDUUKBXTEOFITR-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Ações bioquímicas/fisiológicas
α-Methyl-DL-serine is an amino acid derivative.
Código de classe de armazenamento
13 - Non Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
I S Mendelson
Journal of mental deficiency research, 26 (Pt 2), 107-110 (1982-06-01)
An inherited defect in the glycine cleavage enzyme results in the condition of neonatal glycine encephalopathy which has not responded to the current innovative methods of therapy. A re-examination of the enzyme structure and metabolic pathways, leads us to recommend
H Mickos et al.
Acta chemica Scandinavica (Copenhagen, Denmark : 1989), 46(10), 989-993 (1992-10-01)
The three-dimensional structure of the RGD-adhesion sequence has been studied previously by means of linear and cyclic peptides. These peptides show widely differing affinities to integrins, ascribed to a strong dependence on steric factors in the receptor recognition. Insertion of
Ewa Zabłotna et al.
Biochemical and biophysical research communications, 340(3), 823-828 (2005-12-29)
In many complexes formed by serine proteinases and their inhibitors, the hydroxyl group provided by water molecule or by the inhibitor Ser residue is located close to the inhibitor P1-P1' reactive site. In order to investigate the role of this
Francisco Corzana et al.
Chemical communications (Cambridge, England), 47(18), 5319-5321 (2011-04-01)
A novel Tn antigen mimic, in which the natural underlying amino acid has been replaced by the non-natural α-methylserine analogue, is reported. This derivative exhibits a similar affinity for a natural lectin as for the natural Tn and retains the
An approach to enantioselective activation of N-benzoyl-alpha-methylserine with chiral N-triazinylammonium chloride.
Beata Kolesińska et al.
Acta poloniae pharmaceutica, 63(5), 426-429 (2007-03-16)
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica