M6001
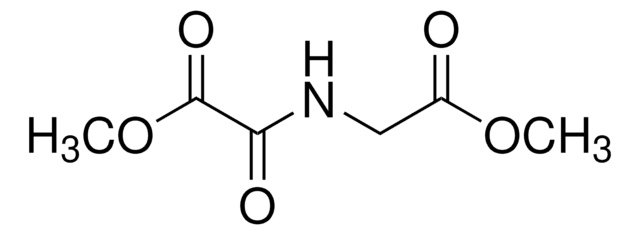
α-Methyl-DL-aspartic acid
Sinônimo(s):
2-Amino-2-methylsuccinic acid
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C5H9NO4
Número CAS:
Peso molecular:
147.13
Número MDL:
Código UNSPSC:
12352209
ID de substância PubChem:
NACRES:
NA.26
Produtos recomendados
Ensaio
≥98% (TLC)
forma
powder
cor
white
cadeia de caracteres SMILES
CC(N)(CC(O)=O)C(O)=O
InChI
1S/C5H9NO4/c1-5(6,4(9)10)2-3(7)8/h2,6H2,1H3,(H,7,8)(H,9,10)
chave InChI
CWAYDJFPMMUKOI-UHFFFAOYSA-N
Ações bioquímicas/fisiológicas
α-methyl-dl-aspartic acid is an iinhibitor of endothelial NO and l-citrulline production.
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Xuejun Zhu et al.
Physical review letters, 108(12), 128101-128101 (2012-05-01)
We study Escherichia coli chemotaxis behavior in environments with spatially and temporally varying attractant sources by developing a unique microfluidic system. Our measurements reveal a frequency-dependent chemotaxis behavior. At low frequency, the E. coli population oscillates in synchrony with the attractant.
Harm Maarsingh et al.
European journal of pharmacology, 546(1-3), 171-176 (2006-08-22)
Nitric oxide synthase (NOS) converts L-arginine into nitric oxide (NO) and L-citrulline. In NO-producing cells, L-citrulline can be recycled to L-arginine in a two-step reaction involving argininosuccinate synthase (ASS) and -lyase (ASL). In guinea pig trachea, L-arginine is a limiting
Intrinsic deuterium kinetic isotope effects in glutamate mutase measured by an intramolecular competition experiment.
Miri Yoon et al.
Angewandte Chemie (International ed. in English), 46(44), 8455-8459 (2007-10-03)
S Rhee et al.
The Journal of biological chemistry, 272(28), 17293-17302 (1997-07-11)
Two high resolution crystal structures of cytosolic aspartate aminotransferase from pig heart provide additional insights into the stereochemical mechanism for ligand-induced conformational changes in this enzyme. Structures of the homodimeric native structure and its complex with the substrate analog 2-methylaspartate
J Jäger et al.
Journal of molecular biology, 239(2), 285-305 (1994-06-03)
Three crystal structures of wild type E. coli aspartate aminotransferase (E.C.2.6.1.1) in space group P2(1) have been determined at resolution limits between 2.6 and 2.35 A. The unliganded enzyme and its complexes with the substrate analogues maleate and 2-methylaspartate resulted
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica