PHR1286
Gemfibrozil
Pharmaceutical Secondary Standard; Certified Reference Material
Sinônimo(s):
2,2-Dimethyl-5-(2,5-dimethylphenoxy)pentanoic acid, 2,2-Dimethyl-5-(2,5-xylyloxy)valeric acid, 5-(2,5-Dimethylphenoxy)-2,2-dimethylpentanoic acid
About This Item
Produtos recomendados
grau
certified reference material
pharmaceutical secondary standard
Nível de qualidade
Agency
traceable to BP 363
traceable to Ph. Eur. Y0000513
traceable to USP 1288500
família API
gemfibrozil
Certificado de análise (CofA)
current certificate can be downloaded
técnica(s)
HPLC: suitable
gas chromatography (GC): suitable
aplicação(ões)
pharmaceutical (small molecule)
formato
neat
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
Cc1ccc(C)c(OCCCC(C)(C)C(O)=O)c1
InChI
1S/C15H22O3/c1-11-6-7-12(2)13(10-11)18-9-5-8-15(3,4)14(16)17/h6-7,10H,5,8-9H2,1-4H3,(H,16,17)
chave InChI
HEMJJKBWTPKOJG-UHFFFAOYSA-N
Informações sobre genes
human ... PPARA(5465)
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Gemfibrozil is a cholesterol-lowering drug that effectively lowers the serum cholesterol, triglyceride, and low-density lipoprotein (LDL) levels. It also efficiently raises the serum high-density lipoprotein (HDL) levels. It is known to minimize the incidence of coronary heart diseases in humans.
Aplicação
Nota de análise
Outras notas
Nota de rodapé
produto relacionado
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Oral
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Choose from one of the most recent versions:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica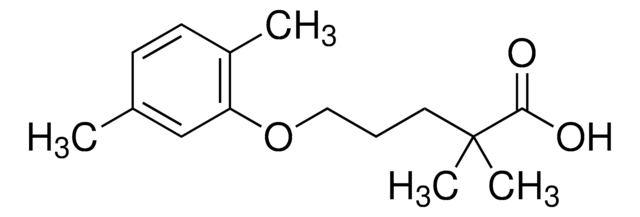



![2-[4-(4-Chlorobenzoyl)phenoxy]-2-methylpropionic acid 95%](/deepweb/assets/sigmaaldrich/product/structures/779/056/45779a0b-0c78-49b4-895b-eae07474ee2e/640/45779a0b-0c78-49b4-895b-eae07474ee2e.png)