88789
Formaldehyde dimethyl acetal
analytical standard
Sinônimo(s):
Dimethoxymethane, Methylal
About This Item
Produtos recomendados
grau
analytical standard
Nível de qualidade
Ensaio
≥99.5% (GC)
prazo de validade
limited shelf life, expiry date on the label
técnica(s)
HPLC: suitable
gas chromatography (GC): suitable
índice de refração
n20/D 1.354
p.e.
41-43 °C
densidade
0.860 g/mL at 20 °C (lit.)
aplicação(ões)
cleaning products
cosmetics
environmental
flavors and fragrances
food and beverages
personal care
Formato
neat
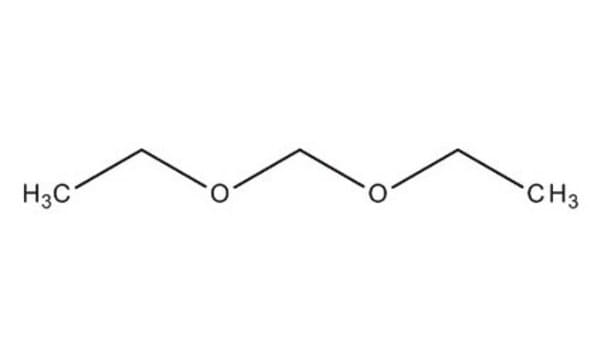
cadeia de caracteres SMILES
COCOC
InChI
1S/C3H8O2/c1-4-3-5-2/h3H2,1-2H3
chave InChI
NKDDWNXOKDWJAK-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Flam. Liq. 2
Perigos de suplementos
Código de classe de armazenamento
3 - Flammable liquids
Classe de risco de água (WGK)
WGK 1
Ponto de fulgor (°F)
-0.4 °F - closed cup
Ponto de fulgor (°C)
-18 °C - closed cup
Equipamento de proteção individual
Eyeshields, Faceshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Active Filters
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica