240877
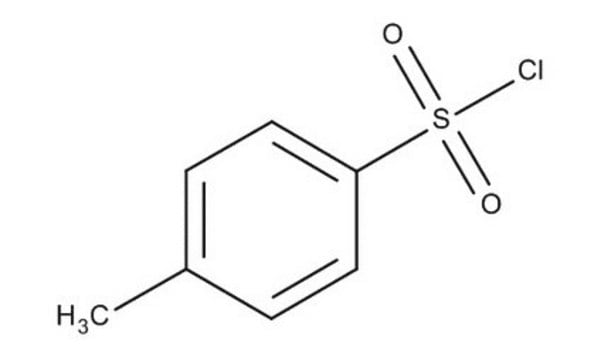
p-Toluenesulfonyl chloride
ReagentPlus®, ≥99%
Sinônimo(s):
TsCl, Tosyl chloride
About This Item
Produtos recomendados
pressão de vapor
1 mmHg ( 88 °C)
Nível de qualidade
linha de produto
ReagentPlus®
Ensaio
≥99%
forma
solid
pb
134 °C/10 mmHg (lit.)
pf
65-69 °C (lit.)
solubilidade
benzene: freely soluble(lit.)
chloroform: freely soluble(lit.)
ethanol: freely soluble(lit.)
water: insoluble(lit.)
cadeia de caracteres SMILES
Cc1ccc(cc1)S(Cl)(=O)=O
InChI
1S/C7H7ClO2S/c1-6-2-4-7(5-3-6)11(8,9)10/h2-5H,1H3
chave InChI
YYROPELSRYBVMQ-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
- In combination with N-methylimidazole for the esterification or thioesterification of carboxylic acids and alcohols or thiols.
- As an additive to enhance the yield of symmetrical biaryls via palladium chloride catalyzed homo-coupling of arylboronic acids in the absence of ligands.
- As a positive chlorine source for the ?-chlorination of ketones.
- Solvent-free tosylation of alcohols and phenols in the presence of heterodoxy acids.
- As an activator for reaction between 2-alkynylbenzaldoxime and phenols to form 1-aroxyisoquinolines in the presence of silver triflate.
- As a catalyst for the solvent-free preparation of symmetrical bis(benzhydryl)ethers from benzhydrols.
Informações legais
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Dam. 1 - Met. Corr. 1 - Skin Irrit. 2 - Skin Sens. 1
Código de classe de armazenamento
8B - Non-combustible corrosive hazardous materials
Classe de risco de água (WGK)
WGK 1
Ponto de fulgor (°F)
262.4 °F - closed cup
Ponto de fulgor (°C)
128 °C - closed cup
Equipamento de proteção individual
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
Click chemistry, and the copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) in particular, is a powerful new synthetic tool in polymer chemistry and material science.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica