H8706
Hexafluorobenzene
99%
Sinônimo(s):
Perfluorobenzene
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C6F6
Número CAS:
Peso molecular:
186.05
Beilstein:
1683438
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
99%
Formulário
liquid
índice de refração
n20/D 1.377 (lit.)
p.e.
80-82 °C (lit.)
pf
3.7-4.1 °C (lit.)
densidade
1.612 g/mL at 25 °C (lit.)
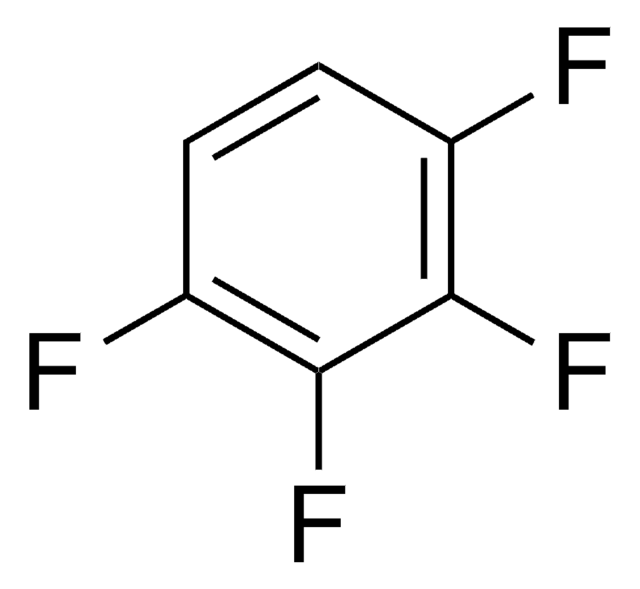
cadeia de caracteres SMILES
Fc1c(F)c(F)c(F)c(F)c1F
InChI
1S/C6F6/c7-1-2(8)4(10)6(12)5(11)3(1)9
chave InChI
ZQBFAOFFOQMSGJ-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Aplicação
Hexafluorobenzene can react with:
It can be used:
- Ethyl magnesium bromide in the presence of transition metal halides to form the corresponding perfluoroarylmagnesium compound that can undergo Grignard reactions.
- The sodium salt of the appropriate phenol in 1,3-dimethyl-2-imidazolidinone (DMEU) to form the corresponding hexakis(aryloxy)benzenes.
It can be used:
- As a ligand to synthesize novel ruthenium(0) and osmium(0) hexafluorobenzene complexes.
- As a solvent and promoter for the ring-closing metathesis (RCM) to form tetrasubstituted olefins in the presence of a ruthenium-based catalyst.
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Flam. Liq. 2
Código de classe de armazenamento
3 - Flammable liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
50.0 °F - closed cup
Ponto de fulgor (°C)
10 °C - closed cup
Equipamento de proteção individual
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Synthesis of hexakis (aryloxy) benzenes: x-ray analysis of hexakis (phenyloxy) benzene and of the acetonitrile clathrate of hexakis (3, 5-dimethylphenyloxy) benzene
Gilmore C J, et al.
Tetrahedron Letters, 24(31), 3269-3272 (1983)
Synthesis of new ? 4-hexafluorobenzene complexes of ruthenium and osmium from atoms of the metals: crystal structure of [Ru (? 6-C 6 H 3 Me 3-1, 3, 5)(? 4-C 6 F 6)]
Martin A, et al.
Journal of the Chemical Society, (15), 2251-2255 (1994)
Markus Allesch et al.
The journal of physical chemistry. B, 111(5), 1081-1089 (2007-02-03)
We report on the aqueous hydration of benzene and hexafluorobenzene, as obtained by carrying out extensive (>100 ps) first principles molecular dynamics simulations. Our results show that benzene and hexafluorobenzene do not behave as ordinary hydrophobic solutes, but rather present
Mariana Palma et al.
Frontiers in physiology, 11, 205-205 (2020-04-09)
Practical diets for commercial barramundi production rarely contain greater than 10% starch, used mainly as a binding agent during extrusion. Alternative ingredients such as digestible starch have shown some capacity to spare dietary protein catabolism to generate glucose. In the
M Albertí et al.
The journal of physical chemistry. A, 115(40), 10871-10879 (2011-09-03)
The effect of some leading intermolecular interaction components on specific features of weakly bound clusters involving an aromatic molecule, a closed shell ion, and Ar atoms is analyzed by performing molecular dynamics simulations on potential energy surfaces properly formulated in
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica