939358
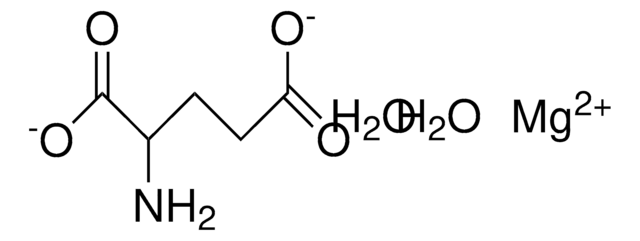
Magnesium acetate tetrahydrate
≥99.9% trace metals basis
Sinônimo(s):
Magnesium Diacetate Tetrahydrate,, Magnesium diethanoate tetrahydrate, Acetic acid magnesium salt
About This Item
Produtos recomendados
grau
for analytical purposes
Nível de qualidade
tipo
(High purity salts)
Ensaio
≥99.9% trace metals basis
98-102% (EDTA, complexometric)
Formulário
powder or crystals
solid
Impurezas
<1000 ppm trace metal basis
cor
white to off-white
pf
72-75 °C (lit.)
72-75 °C
solubilidade
water: soluble
densidade
1.454 g/cm3
traços de ânion
chloride (Cl-): ≤20 ppm
sulfate (SO42-): <50 ppm
traços de cátion
Al: <50 ppm
K: <50 ppm
Mg: <100 ppm
Na: <50 ppm
Pb: <50 ppm
Zn: <50 ppm
aplicação(ões)
battery manufacturing
cadeia de caracteres SMILES
O.O.O.O.CC(=O)O[Mg]OC(C)=O
InChI
1S/2C2H4O2.Mg.4H2O/c2*1-2(3)4;;;;;/h2*1H3,(H,3,4);;4*1H2/q;;+2;;;;/p-2
chave InChI
XKPKPGCRSHFTKM-UHFFFAOYSA-L
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
- A key components for the synthesis of spinel magnesium manganese oxide (Mg0.5Mn2.5O4 ) through one-step colloidal synthesis method. The nanocrystals has exhibited significant electrochemical activities in presence of diverse electrolytes.[1]
- A starting materials for the production of Mesoporous (ZnO)x(MgO)1−x nanoplates by a template-free solvothermal synthetic method followed by subsequent calcination. These materials exhibit a band gap resulting from the presence of ZnO and MgO. The broad peaks observed in the 400-700 nm range indicate the presence of oxygen vacancy defects on the surface of the (ZnO)x(MgO)1−x nanoplates. These nanocrystals showed superior photocatalytic activities for the degradation of methyl orange (MO) in aqueous solution.[2]
- To the synthesis of hydrophobic antireflective films of MgF2 with silicon modified with enhenced durability through sol-gel method.[3]
- As a material for synthesizing carbon nanoribbons using ferrocene at high temperatures. The resulting nanoribbons exhibit a remarkably high surface area and demonstrate a stable reversible capacity of 750 mA h g−1 after 300 cycles in a charge-discharge experiment conducted at 0.5 A g−1. Due to these properties, it would be highly beneficial as an electrode material in electronic devices. [4]
Características e benefícios
Medium purity (99.9%)
Low trace metals in ppm level
Cost effective
Low Chloride and sulfate levels
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 1
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Certificados de análise (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Se precisar de ajuda, entre em contato Atendimento ao cliente
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica