75760
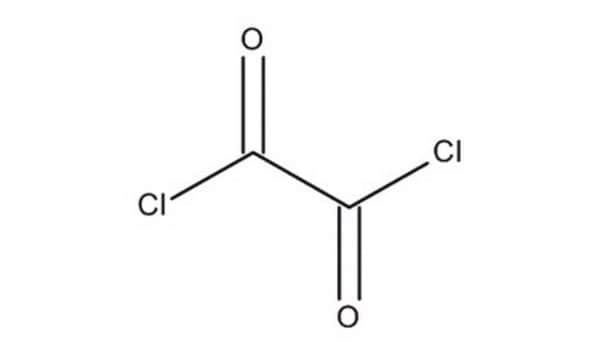
Oxalyl chloride
dist., ≥96.0% (AT)
Sinônimo(s):
Ethanedioyl dichloride
About This Item
Produtos recomendados
densidade de vapor
4.4 (vs air)
pressão de vapor
150 mmHg ( 20 °C)
Ensaio
≥96.0% (AT)
Formulário
liquid
qualidade
dist.
adequação da reação
reagent type: oxidant
índice de refração
n20/D 1.429 (lit.)
p.e.
62-65 °C (lit.)
pf
−10-−8 °C (lit.)
densidade
1.5 g/mL at 20 °C (lit.)
cadeia de caracteres SMILES
ClC(=O)C(Cl)=O
InChI
1S/C2Cl2O2/c3-1(5)2(4)6
chave InChI
CTSLXHKWHWQRSH-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
- Synthesis of N-heterocyclic ynones and ynediones, used to activate carboxylic acids
- Chlorination and halogenation
- Three-component [3+2] cycloadditions
- Reactions with organostannanes
- Synthesis of cyclopentenones
- Carbonylations, used as a carbonyl synthon
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Eye Dam. 1 - Skin Corr. 1B - Water-react 1
Perigos de suplementos
Código de classe de armazenamento
4.3 - Hazardous materials which set free flammable gases upon contact with water
Classe de risco de água (WGK)
WGK 2
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Faceshields, Gloves, Goggles
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Active Filters
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica