706531
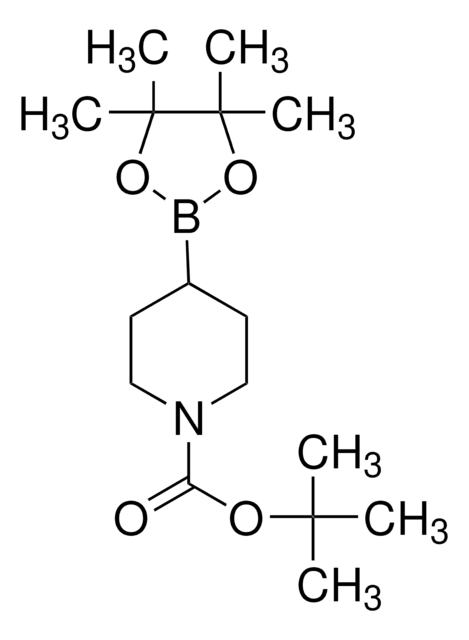
N-Boc-1,2,3,6-tetrahydropyridine-4-boronic acid pinacol ester
95%
Sinônimo(s):
(1-tert-Butoxycarbonyl-1,2,3,6-tetrahydropyridin-4-yl)boronic acid pinacol ester, (N-tert-Butoxycarbonyl-1,2,3,6-tetrahydropyridin-4-yl)boronic acid pinacol ester, (N-tert-Butoxycarbonyl-1,2,5,6-tetrahydropyridin-4-yl)boronic acid pinacol ester, (N-tert-Butoxycarbonyl)-1,2,3,6-tetrahydropyridine-4-boronic acid pinacol ester
Selecione um tamanho
Selecione um tamanho
About This Item
Produtos recomendados
Nível de qualidade
Ensaio
95%
Formulário
powder
pf
100-114 °C
cadeia de caracteres SMILES
CC(C)(C)OC(=O)N1CCC(=CC1)B2OC(C)(C)C(C)(C)O2
InChI
1S/C16H28BNO4/c1-14(2,3)20-13(19)18-10-8-12(9-11-18)17-21-15(4,5)16(6,7)22-17/h8H,9-11H2,1-7H3
chave InChI
VVDCRJGWILREQH-UHFFFAOYSA-N
Categorias relacionadas
Descrição geral
Aplicação
- Suzuki-Miyaura cross-coupling using palladium phosphine catalyst[1]
- Palladium-catalyzed ligand-controlled regioselective Suzuki coupling[2]
- Palladium-catalyzed Suzuki-Miyaura coupling[3]
- Suzuki coupling followed by iodolactonization reaction[4]
- Wrenchnolol derivative optimized for gene activation in cells[5]
Reagent used in Preparation of several enzymatic inhibitors and receptor ligands
- Orally active anaplastic lymphoma kinase inhibitors[6]
- Oxazolecarboxamides as diacylglycerol acyltransferase-1 inhibitors for treatment of obesity and diabetes[7]
- 4-arylpiperidinyl amides and N-arylpiperidin-3-yl-cyclopropanecarboxamides as novel melatonin receptor ligands[8]
- Quinazoline analogs as glucocerebrosidase inhibitors with chaperone activity for treatment of Gaucher disease, a lysosomal storage disorder[9]
- Arylpiperazine and piperidine ethers as dual acting norepinephrine reuptake inhibitors and 5-HT1A partial agonists[10]
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Active Filters
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica

![[Pd(OAc)2]3 reagent grade, 98%](/deepweb/assets/sigmaaldrich/product/structures/508/249/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f/640/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f.png)



![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)

