556017
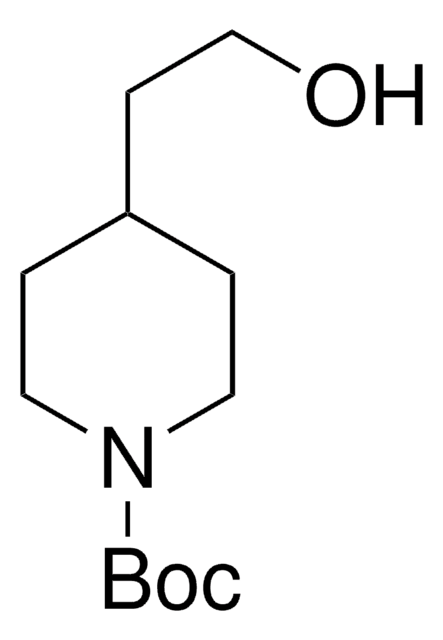
N-Boc-4-piperidinemethanol
97%
Sinônimo(s):
N-tert-Butyloxycarbonyl-4-piperidinemethanol
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C11H21NO3
Número CAS:
Peso molecular:
215.29
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
97%
Formulário
solid
pf
78-82 °C (lit.)
grupo funcional
hydroxyl
cadeia de caracteres SMILES
CC(C)(C)OC(=O)N1CCC(CO)CC1
InChI
1S/C11H21NO3/c1-11(2,3)15-10(14)12-6-4-9(8-13)5-7-12/h9,13H,4-8H2,1-3H3
chave InChI
CTEDVGRUGMPBHE-UHFFFAOYSA-N
Descrição geral
N-Boc-4-piperidinemethanol contains a tert-butyloxycarbonyl (t-BOC)-protecting group. It can be synthesized from 4-piperidinemethanol via reaction with di-tert-butyldicarbonate.
Aplicação
N-Boc-4-piperidinemethanol may be used to synthesize:
- methyl 5-methoxy-4-(1-methylpiperidin-4-ylmethoxy)-2-nitrobenzoate
- N-Boc-4-piperidinecarboxaldehyde
- [1-(tert-butoxycarbonyl)piperidin-4-yl]methyl methanesulfonate
Involved in the synthesis of bradycardic agents.
Código de classe de armazenamento
13 - Non Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Synthesis and nanostructures of 5, 10, 15, 20-tetrakis (4-piperidyl) porphyrin.
JacobsenJL, et al.
Tetrahedron, 69(48), 10507-10515 (2013)
Hideki Kubota et al.
Bioorganic & medicinal chemistry letters, 14(12), 3049-3052 (2004-05-20)
A series of piperidinoalkanoyl-1,2,3,4-tetrahydroisoquinoline derivatives were synthesized, and their bradycardic activities were investigated in the isolated right atria of guinea pigs and in conscious rats. These efforts identified the achiral compound 2f, which exhibited potent and long-lasting bradycardic activity with
Fluorine-18 labeling of 6, 7-disubstituted anilinoquinazoline derivatives for positron emission tomography (PET) imaging of tyrosine kinase receptors: synthesis of 18F-Iressa and related molecular probes.
Seimbille Y, et al.
Journal of Labelled Compounds & Radiopharmaceuticals, 48(11), 829-843 (2005)
Daniele Zampieri et al.
European journal of medicinal chemistry, 44(1), 124-130 (2008-04-29)
We describe here the synthesis and the binding interaction with sigma(1) and sigma(2) receptors of a series of new benzo[d]oxazol-2(3H)-one derivatives variously substituted on the N-benzyl moiety. The results of binding studies confirm the notion that the benzoxazolone moiety confers
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica